Abstract
Aims
Increased sympathetic activation and reduced parasympathetic tone are important pathophysiological contributors to the progression of heart failure, and are associated with poor outcome in patients. The aim of this study is to determine if vagal nerve stimulation (VNS) is a promising approach to modulate autonomic function and slow cardiac remodelling and the progression of heart failure.
Methods
The NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) trial is designed to evaluate whether the Boston Scientific VNS device is safe and may attenuate cardiac remodelling, improve cardiac function and increase exercise capacity, in symptomatic heart failure patients (New York Heart Association Class II–III) with left ventricular systolic dysfunction (ejection fraction ≤35%) and receiving optimal medical therapy. Patients will be randomized in a 2:1 ratio to receive standard optimal medical treatment plus VNS system in an active mode vs. optimal medical treatment plus VNS system in an inactive mode, for a 6 month period. After the 6 month control period, inactive VNS systems will be activated and all patients will receive VNS. The study is powered to detect differences in the primary efficacy endpoint of change in left ventricular end systolic diameter. Secondary endpoints include ejection fraction, left ventricular volumes, quality of life scores, functional capacity, and changes in biomarkers.
Conclusion
This Phase II, randomized clinical trial conducted with vagal stimulation for heart failure will provide important new information on the potential of this novel and promising technique.
Keywords: Autonomic, Cardiac, Device, Heart, Parasympathetic, Vagal
Introduction
Congestive heart failure is a syndrome that affects nearly 23 million people worldwide, with approximately 2 million new patients diagnosed annually.1 Despite substantial advances over the past two decades in pharmacological therapy and device therapy,2 heart failure remains one of the leading causes of morbidity and mortality. Increased sympathetic activation and reduced parasympathetic tone (as reflected by reduced baroreflex sensitivity and/or decreased heart rate variability) are important pathophysiological contributors to the progression of heart failure (HF) irrespective of aetiology, and are associated with poor outcome in patients with congestive heart failure.3,4 Reducing sympathetic drive with anti-adrenergic pharmacological agents has shown to be beneficial. Augmenting the parasympathetic tone via direct vagal stimulation was recently assessed in a non-randomized observational study of 32 HF patients with left ventricular (LV) systolic dysfunction suggesting that vagal stimulation was safe and might favourably influence LV remodelling.5,6
The NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) trial is designed to evaluate whether right vagal nerve stimulation is safe and might attenuate cardiac remodelling, improve cardiac function, and increase exercise capacity in symptomatic HF patients with LV systolic dysfunction and dilation, receiving optimal medical therapy.
Study design
Inclusion criteria:
Age 18 years or above, and of legal age to give informed consent specific to national laws.
Willing and capable of providing informed consent.
Capable of participating in all testing associated with this clinical investigation.
Stable symptomatic HF New York Heart Association (NYHA) class III, amended to include NYHA classes II and III.
Left ventricular (LV) ejection fraction <35% (equal or smaller than 35 %) documented in patient file within the past 6 months.
Left ventricular end diastolic diameter (LVEDD) of 5.5 cm or greater documented in patient file within the past 6 months.
Prescribed to optimal pharmacological therapy according to current local or international guidelines for treatment of acute and chronic HF at the time of enrolment, unless contraindicated or not-tolerated, and on a stable dose (e.g. recommended no greater than a 50% increase or decrease in dosage, amended to ‘no greater than a 100% increase or 50% decrease in dosage’) for at least 30 days before enrolment.
Abbreviated exclusion criteria:
Cardiac resynchronization therapy (CRT), subsequently amended to exclude patients with CRT for less than 1 year before enrolment (the total number of CRT patients will not exceed 30).
Patients who have been hospitalized for HF and who required the use of HF IV therapy within 30 days before enrolment.
Patients with unstable angina, myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass graft, cerebral vascular accident, or transient ischaemic attack within previous 3 months (90 days) before enrolment.
Patients whose primary cause of HF is mitral or aortic valve disease, classified as severe valve disease.
Patients with persistent or permanent atrial fibrillation (AF) (according to the current local or international guidelines for the management of patients with AF at the time of enrolment) within 180 days before enrolment, amended to within 90 days before enrolment.
Pacemaker-indicated patients.
Patients actively treated, amended to patients who have started treatment within the previous 6 months before enrolment, for sleep apnoea or sleep-disordered breathing with therapies to maintain airway patency [e.g. continuous positive airway pressure (CPAP), b-iphasic positive airway pressure (BiPAP), automatic positive airway pressure (APAP)] with or without oxygen supplementation.
Patients on or indicated for renal dialysis.
Insulin-dependent diabetic patients, amended to Type 1 diabetic patients and Type 2 diabetic patients that have been treated with insulin for more than 5 years before enrolment.
Patients implanted with any active implantable medical device other than a left-sided single or dual chamber implantable cardioverter defibrillator (ICD) with bipolar sensing, amended to add other than a left-sided CRT device with each sensing channel programmed to either bipolar sensing or no sensing. All enrolled CRT patients must have had CRT for at least 1 year before enrolment.
Boston Scientific vagal nerve stimulation (VNS) system
The Boston Scientific VNS system is investigational. It consists of the investigational vagal cuff lead (NCT Lead; Boston Scientific Corporation, St. Paul, MN, USA) and of an implantable pulse generator (PrecisionTM; Boston Scientific Corporation). The NCT lead (see Figure 1) is a single lead with a bipolar, helical cuff, with an active anode and cathode, as well as a non-active strain relief cuff.
Figure 1.

The NCT lead is for investigational use only.
Investigational therapy
The investigational vagal cuff lead is implanted in all patients by exposing approximately 4–6 cm of the vagus nerve on the right side of the neck. The lead is then tunnelled over the clavicle and connected to a pulse generator (Figure 2). The procedure can be performed under general or local anaesthesia by an appropriately trained and experienced vascular/cardiovascular surgeon or neurosurgeon. Although the recommendation is to use general anaesthesia, the surgical team may decide to use local anaesthesia if there is concern about patient safety. Based upon the clinical experience of vagal nerve implants to treat epilepsy, the procedure will take 90 minutes or less to implant in most cases.
Figure 2.
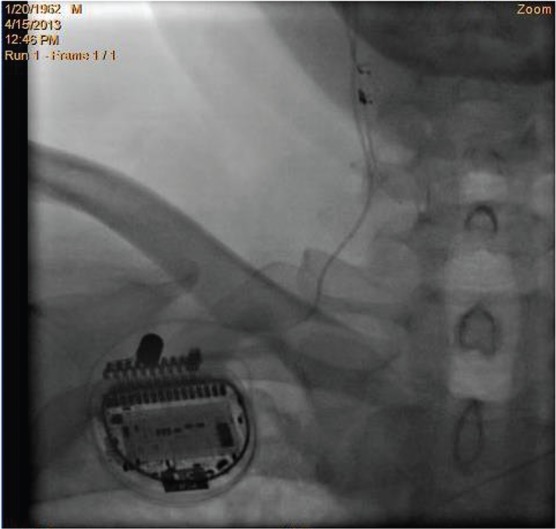
The NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) implant location. The lead is placed around the cervical vagus nerve and then tunnelled across the clavicle and connected to the pulse generator, which is located in a pectoral pocket.
Stimulation testing and therapy titration
Once implanted, the system is evaluated by checking the impedance of both electrodes, and then by performing brief stimulation to look for one or more of the following as evidence of proper system functioning and implant location: activation of the larynx, an acute heart rate reduction of greater than 5 beats, or a respiratory response (breath reflex). For patients assigned to the active treatment group, therapy is delivered at 20 Hz for 10 s out of every minute. Titration is performed in all patients, by increasing the current amplitude (mA) slowly to allow for accommodation of anticipated side-effects, such as hoarse voice, a tickling sensation in the throat, brief cough, or muscle fasciculation that may occur at higher stimulation. The goal is to titrate patients to the maximum tolerable dose, not to exceed 8 mA during the implant and not exceeding 4 mA chronically.
Study procedures
Figure 3 shows the flow of the study visits.
Figure 3.
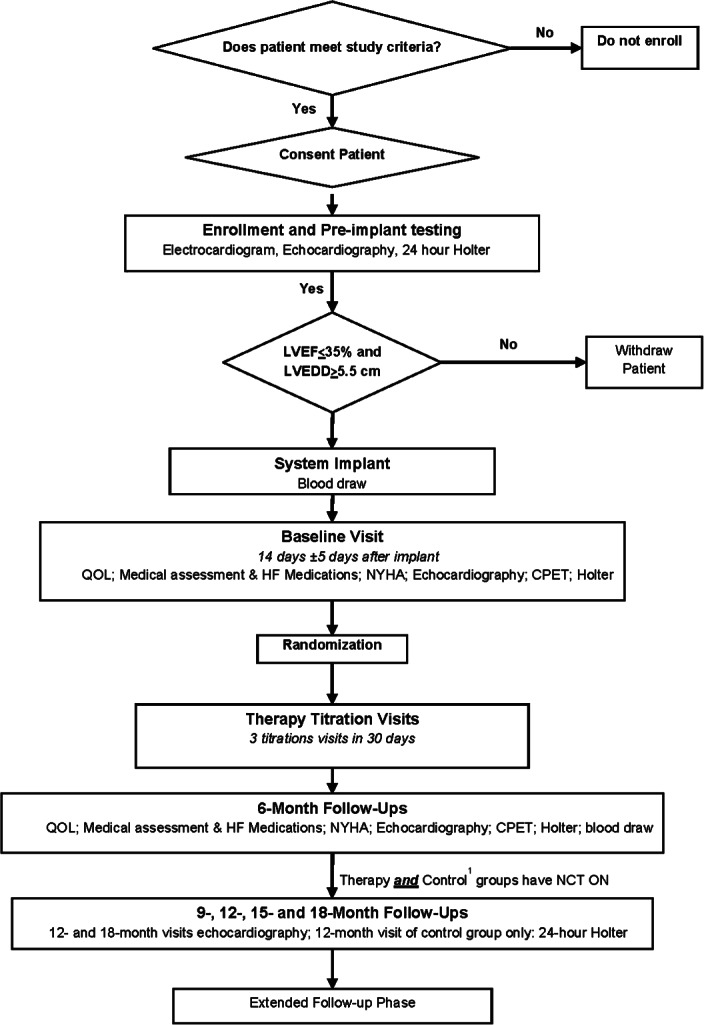
Study flow chart and data collection. CPET, cardiopulmonary exercise test; HF, heart failure; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; QOL, quality of life.
Screening and baseline evaluation
Following confirmation of inclusion/exclusion criteria and patient consent, pre-implant screening is performed to confirm the eligibility criteria, including left ventricular ejection fraction (LVEF) ≤35% and LVEDD >5.5 cm. All eligible patients are scheduled for implantation of the Boston Scientific VNS system. At 14 ± 5 days after implant patients return for the collection of baseline data (Figure 3). Medical assessment, NYHA class status assessment, echocardiography and cardiopulmonary exercise testing are performed by investigators blinded to treatment assignment. Data are uploaded electronically to an internet-based electronic data capture system and sent to the core laboratories for blinded analysis.
Randomization
At the end of the baseline visit, patients are randomized 2:1 to therapy or control in permuted blocks within each centre, stratified by clinical site. Using randomization blocks conceals treatment allocation. The randomization is performed using an electronic database at the time of the baseline visit.
Blinding
Following randomization, all patients undergo the first of four titrations (including the baseline visit) during a 30 day window (Figure 3). Although every attempt is made to blind treatment assignment, many patients feel the stimulation as a slight tickling/vibration in the neck. Thus, complete blinding is not feasible. However, assessment of all endpoint-related data are performed by blinded clinicians, protecting data integrity from treatment assignment bias. At the 6 month follow-up, patients that were randomized to the control group will be crossed over to begin receiving therapy. They will also receive an additional three titrations during the first 30 days following the 6 month visit. This ensures optimal programming of the VNS system.
Follow-up
There are two phases to the study. The primary study phase consists of approximately 19 months of follow-up visits after implant for each patient. These 19 months include the 30 days needed for titration after baseline and the following 18 months of follow-up time with regularly reoccurring visits. Following the fourth titration after implant, patients return to the clinic at 3 months for a device check and therapy titration, and then again at 6 months for collection of all efficacy-related endpoint data (Figure 3). After this patients return to the clinic every 3 months during a 12 month period of safety follow-up. As patients complete the primary study phase, they enter the extended follow-up phase and continue to receive therapy up to the end of the trial, or indefinitely if is not stopped prematurely for safety concerns. Once all patients complete the primary study phase, a data safety monitoring committee (DSMC) will convene and make a recommendation on whether to continue with active therapy or to terminate the study and deactivate devices.
Efficacy endpoint
Preclinical evidence indicated that a 10% reduction in left-ventricular end systolic diameter (LVESD) is achievable with vagal stimulation.7 Thus, we used a change of 6 mm in LVESD from baseline to 6 months for statistical calculations. With a 2:1 randomization, a power of 80% at minimum, and accounting for patient and data attrition of up to 40%, the required number of patients to receive the Boston Scientific VNS system has been estimated to be 96 patients. To assess the primary efficacy endpoint of LVESD, analysis will be carried out on a modified intention-to-treat basis as specified in the NECTAR-HF protocol. Only patients with a complete baseline and 6 month echo data sets will be analysed. However, a strict intention-to-treat analysis will also be performed, including all patients, regardless of whether they have a complete echo data set or not. For all other efficacy endpoints patients must have a complete data set for the variable being considered for inclusion in the analysis [e.g. peak oxygen consumption (VO2), quality of life (QOL)]. To test the hypothesis for the primary endpoint a general linear model will be used, including baseline LVESD as a covariate in the model.
Safety endpoint
At the end of the primary study phase (approximately 19 months after baseline, and 18 months of follow-up), a safety endpoint of all-cause mortality will be evaluated. Survival will be estimated using Kaplan–Meier methodology and will be compared with an objective performance criterion (OPC) of 65%, derived from the 75% survival at 18 months observed in the COMPANION8 study control group of NYHA class III patients factoring in a clinically meaningful delta of −10%. Patients will be entered into the Kaplan–Meier risk set at the protocol-defined time of therapy activation. A 95% one-sided lower confidence limit will be calculated for the survival estimate and compared with the OPC of 65%. If the lower limit is at least as large as the OPC, it will be concluded that NECTAR-HF has 95% confidence that survival is at least 65%.
Echocardiography
A cardiac imaging core laboratory (CICL) is used to perform echocardiography analysis for endpoint-related data. The echocardiography examination should be performed before any exercise test, or no sooner than within 2 h of finishing the exercise test. The parasternal long and short axis, and the apical four-, five-, two-, and three-chamber views with and without Doppler assessment are required. An electronic copy [e.g. digital imagining and communications in medicine (DICOM)] of the pre-implant baseline echocardiography and of subsequent follow up echocardiographies (6, 12 and 18 month follow-up visits) is sent to the CICL within five working days. The following echocardiographic assessments are made by the CICL: global ventricular size and function including LVEF, end-diastolic volume and diameter, end-systolic volume, diameter and mass; left atrial size, including left atrial volume; mitral regurgitation severity; and left ventricular diastolic function, including mitral inflow Doppler and mitral annular tissue Doppler parameters. All measurements are made in triplicate. In patients who have developed new atrial fibrillation during the course of the trial, echo measurements should be repeated five times.
Cardiopulmonary exercise test (CPET)
Briefly, the testing protocol consists of an initial rest period (minimum of 3 min) and staged exercise. The workload begins at 25 W and increases 10 W every minute. A peak respiratory exchange ratio of 1.1 is strongly encouraged. A minimum 5 min recovery period follows termination of exercise. The CPET may be conducted on either a treadmill or cycle ergometer but the exercise modality selected for an individual patient must be the same at all CPET examinations. Respiratory gases are analysed using a calibrated metabolic cart capable of recording breath-by-breath data (mixing chamber method is also acceptable). A CPET core laboratory will analyse the CPET data, including peak oxygen consumption, peak respiratory exchange ratio, exercise duration, and work performed. Submaximal functional capacity will be assessed by ventilatory anaerobic threshold (VT). Before an investigational site being authorized to enrol patients in the NECTAR-HF study, a CPET certification needs to be completed, assessing the site’s CPET testing capabilities, such as equipment function and calibration, proper demonstration of a valid maximal exercise test, and data formatting for electronic submission into the database. In order to maintain data quality standards, all sites will be required to recertify if a 6 month period lapses without a CPET of a study patient.
Quality of life assessment
Patients are asked to complete two QOL questionnaires at visits as described in Figure 3. The Medical Outcomes Study Short-Form 36-Item Health Survey (SF-36), which includes 36 items that measure general, physical and mental health, and the Minnesota Living with Heart Failure© (MLHFQ) survey questionnaire evaluating the patient’s perception of how his/her HF and therapy affects his/her life.
Biomarkers
To identify markers predictive of response and to better understand the therapy efficacy, panels of markers will be assessed that may include specific biomarkers associated with heart failure [e.g. BNP, atrial natriuretic peptide (ANP), ST2, mid-regional pro-adrenomedullin (MR-proADM), endothelins, miRNA423], inflammation [e.g. tumour necrosis factor alpha (TNFα), interleukin-6 (IL-6), C-reactive protein (CRP), vascular adhesion protein-1 (VAP-1)], apoptosis [e.g. caspase-3, Fas-ligand (Fas-L)], remodelling [e.g. collagen, N-terminal propeptide of human procollagen type I (PINP), N-terminal propeptide of Type III procollagen (PIIINP), Pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), matrix metalloproteinase-3 (MMP-3), matrix metalloproteinase-9 (MMP-9), Tissue inhibitor of metalloproteinase 1 (TIMP-1)], cardiac stress (copeptin), cardiac contraction [e.g. sarcoendoplasmic reticulum calcium ATPase (SERCA-2), ryanodine receptor], and neuro-hormonal activation [e.g. acetyl choline (Ach), catecholamines, vasoactive intestinal peptide (VIP)].
Oversight committees
A steering committee will oversee the detail conduct of the trial from conception to termination, interpretation of the results, and final publication of the results. A DSMC has been appointed to direct data analyses needed for assessing treatment effects during the trial. Interim reports will be reviewed for evidence of adverse events/effects and, accordingly, will advise the steering committee and the sponsor on any possible changes required in the treatment protocol. A clinical event committee (CEC) has been formed to review and adjudicate reports prepared by the data coordinating group to detect any possible safety issue and/or related deficiencies in data collection if required. The committee is responsible to review the data from four different types of events, including deaths, system failure, system infections, and ICD interaction. The CEC may adjudicate other safety issues as well. A listing of committee members can be found Appendix 1.
Discussion
The restoration/augmentation of parasympathetic tone has recently emerged as a promising therapeutic approach to normalizing autonomic imbalance and inhibiting the progression of HF.
The first experimental study to show the potential of chronic vagal stimulation to improve cardiac function was that of Li et al.9 Rats subjected to a large anterior myocardial infarction (MI) leading to heart failure were randomized 14 days post-MI to sham stimulation or active vagal stimulation for 10 s every minute with intensity adjusted to reduce heart rate by 20–30 bpm from a starting value of 360 bpm. The treated rats had significantly lower LV end-diastolic pressure and higher maximum dp/dt of left ventricular pressure than the untreated rats. Improvement of cardiac pumping function was accompanied by a decrease in normalized biventricular weight and, more strikingly, by a 73% decrease in mortality from 50% to 14% at 140 days.
Zhang et al.10 studied the effects of chronic vagal stimulation delivered at a frequency of 20 Hz, a duty cycle of 14 s on and 12 s off, and an intensity individually titrated to reduce sinus rate by approximately 20% in a canine model of HF induced by high-rate ventricular pacing. The dogs were subjected to 8 weeks of high-rate ventricular pacing with concomitant vagus nerve stimulation in the active group and no stimulation in the control group. Animals in the vagus nerve stimulation group had significantly lower LV end diastolic and end systolic volumes and higher LV ejection fraction. In addition, vagal stimulation significantly attenuated the reduction in baroreflex sensitivity induced by heart failure. These results were obtained in the absence of any heart rate effect provided by vagal stimulation as both groups were subjected to the same constant ventricular pacing.
Sabbah et al.11 have developed a canine model of chronic HF produced by multiple sequential coronary micro-embolizations that has been used for a few studies with vagal stimulation. In the first study, right vagus nerve stimulation synchronized to the cardiac cycle with intensity adjusted with a feedback heart rate control system to reduce baseline heart rate by approximately 10%, decreased LV end systolic and end diastolic volumes and increased LVEF. In the same animal model, it was subsequently shown that the addition of vagal stimulation to beta-blockade increased LVEF and decreased LV end systolic volumes more than obtained by beta-blockade alone12. Finally, vagal stimulation with settings that did not cause any acute heart rate reduction (being therefore lower in intensity compared with the previous studies) decreased LV end systolic volume and increased LVEF by a magnitude grossly similar to that achieved in the previous study with more intense closed-loop stimulation.7 Several biomarkers of HF were also positively affected by vagal stimulation.
Potentially beneficial effects of vagal stimulation, independent and/or beyond heart rate decrease include a comprehensive anti-adrenergic activity at presynaptic, postsynaptic, and central levels, an increase in nitric oxide availability an anti-apoptotic effect as well as anti-inflammatory activity.13 Tracey14 provided clear evidence of the existence of a vagally mediated anti-inflammatory effect that has been confirmed in several experimental studies of myocardial ischemia and reperfusion13. Vagal stimulation has also been found to provide a powerful anti-arrhythmic activity, particularly in the setting of myocardial ischaemia.15,16
The clinical experience consists of one completed multi-centre, open label, non-randomized study of 32 patients with NYHA class II–IV and an ejection fraction of ≤35% implanted with a right vagus nerve stimulation system and followed for up to 12 months. There were significant improvements in the 6 min walk test, quality of life, LVEF and LV volumes, and only two non-serious adverse events occurred, related to the device.6 An important next step is to extend these findings by conducting a randomized, controlled and blinded study (i.e. endpoint evaluation is conducted by blinded personnel). NECTAR-HF is a feasibility and proof of concept study. It is intended to help estimate the efficacy of the Boston Scientific VNS system on the endpoint of LV remodelling and clinical benefit. Subsequently, this must be evaluated in an appropriate outcome trial.
Study population
In phase II proof of concept studies, it is desirable to select a homogeneous experimental group of patients in order to avoid the dilution of the potential efficacy endpoint by inconsistent effects across a heterogeneous pattern of response. Therefore, we chose to enrol only symptomatic heart failure patients with a dilated and dysfunctioning LV on optimal recommended therapy, and remote from an acute event and/or CV procedure. Patients with permanent/persistent atrial fibrillation are also excluded, because it is unclear how VNS may affect this condition, and the echocardiographic determination of the primary efficacy endpoint is less accurate in these patients. Insulin-dependent Type 1 diabetes patients and those with long lasting (>5 years) Type 2 diabetes, as well as other conditions associated with features of autonomic dysfunction, were other important exclusions.
Endpoints
Left ventricular end-systolic diameter has been used to evaluate the efficacy of HF therapies in phase II studies.17 Although change in LV dimensions is a surrogate marker for clinical benefit, it has consistently been shown to predict the effect of HF therapies on cardiovascular events and overall mortality.18 The minimal follow-up period for detectable changes in cardiac remodelling is debatable, but in recent CRT studies, a 6 month follow-up period has been an adequate duration to prove reverse remodelling and improvement of LV function.19,20 In NECTAR-HF all other LV dimensions will be examined within the context of changes in a host of secondary efficacy endpoints. The impact of vagal stimulation on quality of life, functional capacity, and all-cause or HF hospitalizations will also be examined to determine if therapy provided a clinical benefit. Furthermore, changes assessed in biological markers will help to support a potential benefit of increased vagus activity.
Control group and blinding
A randomized controlled experimental design is an essential step in medical progress. Although sham surgery may be felt as being a violation of regulatory and ethical principles with regard to risk to patients,21,22 conducting studies without appropriate controls inevitably leads to bias, incorrect interpretation and inconclusive results. Blinding is another challenge in device trials. Stimulating the vagus nerve is known to elicit mild side-effects in many patients, the most prominent of which are voice alteration/throat tingling and coughing. This introduces additional challenges in keeping patients blinded to treatment assignment. In the NECTAR-HF study, we employed a strategy that involves titration of both treatment and control groups, ensuring that patients in both groups have an equal number of visits during the 6 month randomized therapy period. Although patients are not told of their treatment assignment, we do not consider the therapy patients to be blinded. NECTAR-HF is therefore a single-blind study, with only the clinicians involved in data collection, such as echocardiography, exercise testing, and clinical assessment, being blinded to treatment assignment.
Long-term follow up
Switching all patients to the ON mode at the end of the 6 month randomized therapy period exposes the study to the confounding ethical dilemma of implementing an unproven therapy to control patients before the study is completed. However, because vagal stimulation has a well-established safety profile in the epilepsy population with more than 100 000 implants world-wide, it was decided that crossing over the control patients to the ‘ON’ mode carries an acceptable risk.
Additional VNS Studies for Heart Failure
INcrease Of VAgal TonE (INOVATE-HF)23 is a pivotal randomized trial, sponsored by BioControl Medical (Yehud, Israel), examining chronic vagal stimulation, by means of the same device used in the pilot study,6 in HF patients. The patients will be randomized in a 3:2 ratio to receive active treatment after device implant and optimized medical treatment of heart failure (without device implant). The trial has as a primary efficacy endpoint the combination of all-cause mortality and heart failure hospitalisation and intends to enrol 650 patients.
There are potentially important differences between the Boston Scientific VNS system to be tested in NECTAR-HF and the CardioFit device used in the INOVATE-HF trial. First, the CardioFit system intracardiac lead allows the vagus nerve to be stimulated at a certain phase of the cardiac cycle, based upon sensing the R wave whereas the NECTAR-HF will not incorporate heart rate sensing for therapy initiation or timing of the vagus nerve stimulation to the cardiac cycle and will therefore evaluate whether this approach, which avoids the need for an intracardiac lead, will be associated with a clinical benefit. The stimulation lead is also different. The CardioFit stimulation lead is an asymmetric bipolar multicontact cuff electrode specifically designed for cathodic induction of action potentials, while simultaneously applying asymmetrical anodal blocks expected to lead to preferential, but not exclusive, activation of vagal efferent fibres.24 The two electrodes exhibit grossly similar thresholds of activation of A and B fibres;24,25 however, nerve stimulation in the INOVATE-HF study will likely be performed at higher amplitudes (but lower frequencies). Evaluation of the results of the studies may increase our understanding of the mechanisms linking vagus nerve stimulation to clinical benefit.
Finally, a single-arm open label study (ANTEM-HF)26 is currently ongoing, assessing the tolerability and the effects of left sided and right sided vagus nerve stimulation.
Funding
This study is fully sponsored by Boston Scientific Corporation.
Conflicts of interest: G.M.DeF., A.T., J.B., H.K., C.B., D.W., S.S., and F.Z. have received consulting fees for their work on the NECTAR-HF study. S.R., A.R., S.M., B.S., and K.S. are full-time employees of Boston Scientific Corporation.
Appendix 1
Steering Committee: Faiez Zannad, Gaetano De Ferrari, Josep Brugada, Jay Wright, Helmet Klein, John McMurray, Anton Tuinenburg, Christian Butter and Ken Stein
Clinical Events Committee: Daniel Gras, Luigi Tavazi and Dirk De Ridder
Data Safety Monitoring Committee: Henry Dargie, Erland Erdman, Erik Bloch Thomsen, Andreas Shultz Bonhage and Ian Ford
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW. Comprehensive treatment of heart failure: state-of-the-art medical therapy. Rev Cardiovasc Med. 2005;6(Suppl 2):S43–S57. [PubMed] [Google Scholar]
- 3.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure. Clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, Webb-Peploe K, Harrington D, Banasiak W, Wrabec K, Coats AJ. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L, Odero A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ. CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 7.Hamann JJ, Ruble SB, Stolen C, Wang M, Gupta RC, Rastogi S, Sabbah HN. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Fail. 2013;15:1319–1326. doi: 10.1093/eurjhf/hft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 11.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 12.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–178. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 14.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 15.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 16.De Ferrari GM, Vanoli E, Schwartz PJ. Vagal activity and ventricular fibrillation. In: Levy MN, Schwartz PJ, editors. Vagal Control of the Heart: Experimental Basis and Clinical Implications. Armonk, NY: Futura Publishing Co; 1994. pp. 613–636. [Google Scholar]
- 17.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 18.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2000;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA, Lieberman R, Liem LB, O’Connell JB, Schroeder JS, Wheelan KR. Multicenter In Sync ICD II Study Group. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 20.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 21.Freeman TB, Vawter DE, Leaverton PE, Godbold JH, Hauser RA, Goetz CG, Olanow CW. Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson’s disease. N Engl J Med. 1999;341:988–992. doi: 10.1056/NEJM199909233411311. [DOI] [PubMed] [Google Scholar]
- 22.Macklin R. The ethical problems with sham surgery in clinical research. N Engl J Med. 1999;341:992–996. doi: 10.1056/NEJM199909233411312. [DOI] [PubMed] [Google Scholar]
- 23.Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, Mann DL. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J. 2012;163:954–962. doi: 10.1016/j.ahj.2012.03.021. e1. [DOI] [PubMed] [Google Scholar]
- 24.Anholt TA, Ayal S, Goldberg JA. Recruitment and blocking properties of the CardioFit stimulation lead. J Neural Eng. 2011;8:034004. doi: 10.1088/1741-2560/8/3/034004. doi: 10.1088/1741–2560/8/3/034004. [DOI] [PubMed] [Google Scholar]
- 25.Yoo PB, Lubock NB, Hincapie JG, Ruble SB, Hamann JJ. Grill WM (2013) High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J Neural Eng. 2013;10:026003. doi: 10.1088/1741-2560/10/2/026003. doi: 10.1088/1741–2560/10/2/026003. [DOI] [PubMed] [Google Scholar]
- 26.DiCarlo L, Libbus I, Amurthur B, Kenknight BH, Anand IS. Autonomic Regulation Therapy for the Improvement of Left Ventricular Function and Heart Failure Symptoms: The ANTHEM-HF Study. J Card Fail. 2013;19:655–660. doi: 10.1016/j.cardfail.2013.07.002. [DOI] [PubMed] [Google Scholar]


