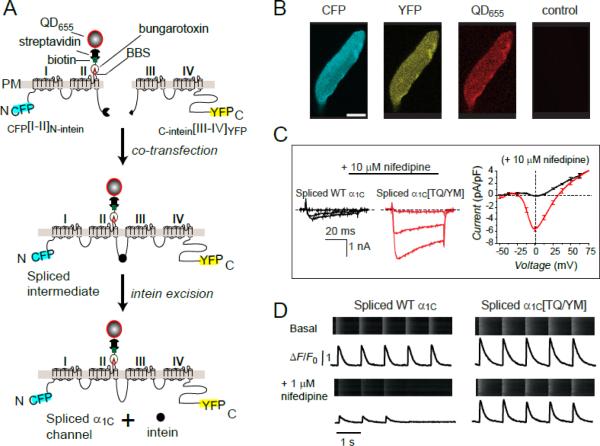
Figure 1. Functional expression of engineered CaV1.2α1C in adult cardiomyocytes using a split-intein ligation strategy.
(A) Strategy for generating full-length α1C from two halves using split-intein protein transsplicing. A 13-residue bungarotoxin binding site (BBS) inserted into the extracellular domain II S5-S6 linker enables detection of surface channels with bungarotoxin-biotin and streptavidin-conjugated quantum dot. (B) Fluorescent detection of split-intein ligated α1C expressed in an adult rat cardiomyocyte. (C) Left and middle, CaV1.2 currents in myocytes expressing either intein-spliced wild-type α1C (black) or a dihydropyridine-resistant mutant, α1C[TQ/YM] (red), in the presence of 10 μM nifedipine. Right, I-V curves in 10 μM nifedipine for intein-spliced WT (black) and DHP-resistant (red) α1C channels with 2 mM Ca2+ as charge carrier. (D) Impact of nifedipine on Ca2+ transients from cardiomyocytes expressing either intein-spliced wild-type (left) or dihydropyridine-resistant (right) α1C.