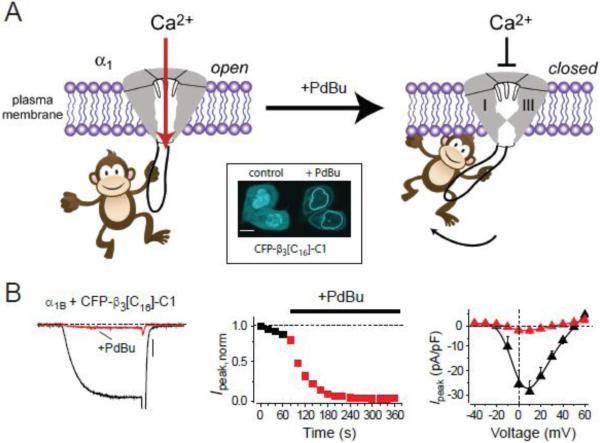
Figure 3. Channel inactivation induced by membrane tethering an associated protein (ChIMP).
(A) Cartoon showing concept of ChIMP. A cytosolic protein associated with a cytoplasmic domain of a CaVα1 subunit is permissive for ionic conductance (left). Directly anchoring the cytosolic protein to the plasma membrane induces a conformational change that closes the channel (right). Inset, phorbol-12,13-dibutyrate (PdBu)-induced membrane translocation of a C-terminus truncated CFP-tagged CaVβ3 fused to the C1 domain of protein kinase C (CFP-β3[C16]-C1). (B) Conversion of CaVβ3 into a PdBu-inducible CaV2.2 channel inhibitor using the ChIMP concept.