Figure 2. In vitro genesis of cancer-associated fibroblasts.
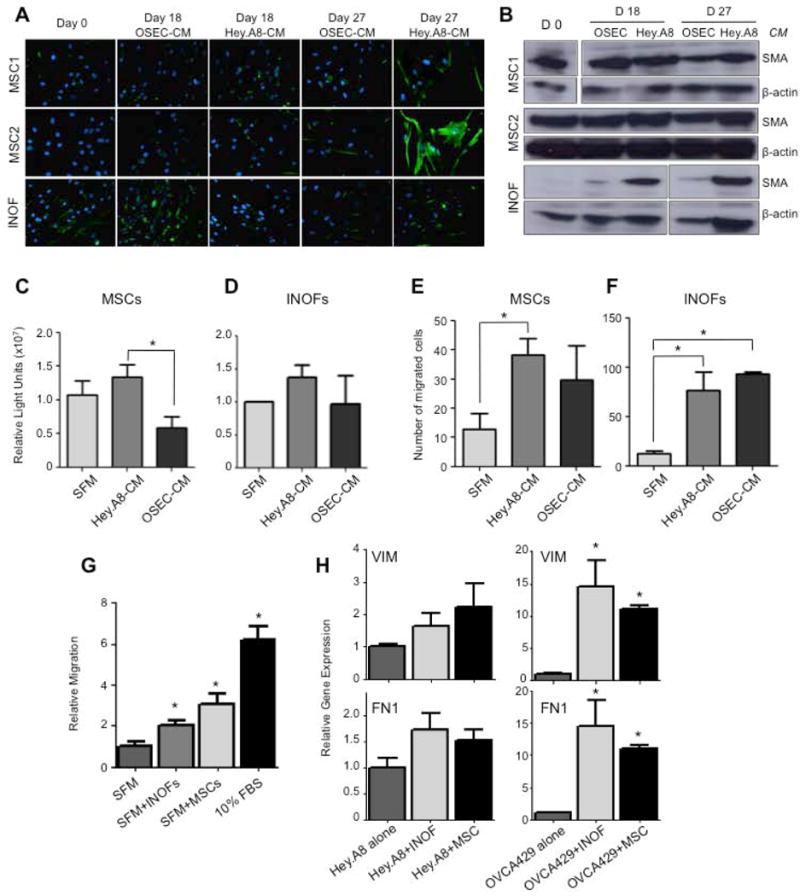
(a) Cancer associated fibroblast precursor cells, mesenchymal stem cells (MSCs, N=2) and immortalized normal ovarian fibroblasts (INOFs, N=1) were cultured in normal ovarian epithelial cell conditioned medium (OSEC-CM) or epithelial ovarian cancer cell conditioned medium (EOC-CM). After 0, 18 and 27 days cells were stained for vimentin (green), nuclei are stained blue with DAPI. MSCs upregulated vimentin when cultured in EOC-CM relative to OSEC-CM treated cells. INOFs already expressed high levels of this marker and no change in expression was observed. (b) After 28 days of culture in EOC-CM, MSCs and in particular INOFs, upregulated expression of α-smooth muscle actin (αSMA), as measured by Western blotting. When cultured in Hey.A8-CM we measured a 1.4-fold and 19.4-fold increase in αSMA expression in MSCs and INOFs respectively (compared to day 0 controls). Beta-actin was used as a loading control. Proliferation assays for (c) MSCs and (d) INOFs cultured in EOC-CM and OSEC-CM; MSCs are significantly more proliferative when cultured in EOC-CM. Migration assays measuring chemotaxis of stromal cells towards conditioned media. (e) MSCs migrate more towards EOC-CM than serum free media or OSEC-CM. (f) INOFs migrate significantly more towards EOC-CM and OSEC-CM than serum-free media. Data shown are mean ± standard deviation (s.d.) for three independent experiments. *P<0.05, two-tailed paired Student's T-test, α=0.05, compared to SFM control. (g) MSCs and INOFs produce secreted factors that promote migration of epithelial ovarian cancer cells. Hey.A8 EOC cells are 2-3 fold more migratory in the presence of INOFs and MSCs compared to control media. 10% fetal bovine serum (FBS) was used as a chemoattractant. (h) EOC cells upregulate mesechymal markers in the presence of co-cultured stromal cells. VIM, vimentin; FN1, fibronectin. *P>0.05, two-tailed paired Student's T-test, α=0.05, compared to EOC cells cultured alone. Error bars = s.d.
