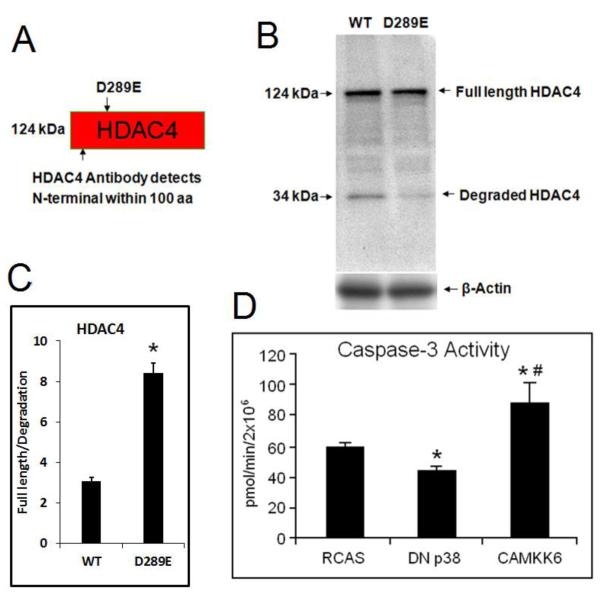
Figure 2. Mutation of Asp-289 stabilized HDAC4 and CaMKK6 induces capase-3 activity in chondrocytes.
(A) Schematic structure of HDAC4 revealed Asp 289 as the target of mutation to remove capase-2 and 3 cleavage site, and HDAC4 antibody detects both N-termimal degradation fragment and full length HDAC4. (B) Western blot shows that HDAC4 is cleaved in WT but the mutant D289 is resistant to degradation of HDAC4. The result indicates that the degradation of HDAC4 is related to ASP-289. (C) Intensity of full length HDAC4/degradation fragment was presented. *, p<0.05. (D) Caspase activity assays showed that over-activation of p38 by CAMKK6 stimulates caspase-3 activity while inhibition of p38 by DN p38 has the opposite effect when comparing to the empty RCAS control. Data are the means of three independent experiments, with S.D. indicated. *, p< 0.05 compared with control. #, p< 0.05 compared with DN p38.