Figure 4.
Enzymology, Cellular Expression, and Homo-oligomerization of Pathogenic Lon Proteins
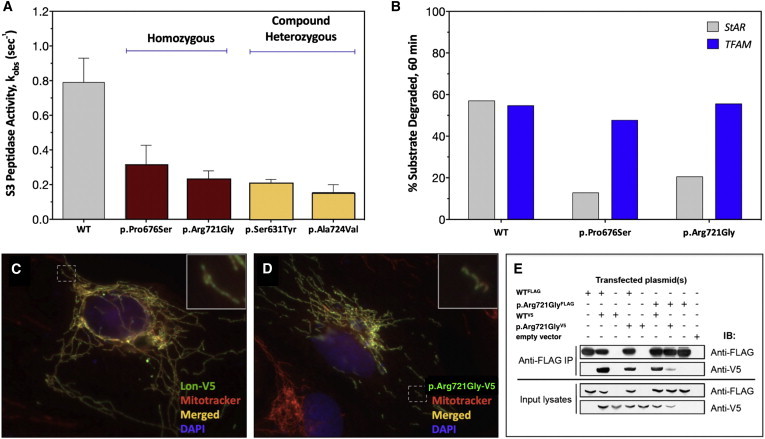
(A) ATP-dependent degradation of the fluorogenic peptide substrate S3. The rates of S3 cleavage were determined with recombinant Lon proteins (250 nM monomer) and 0.5 mM of S3 (0.5-fold Km) in buffer. The degradation reactions were initiated by the addition of Mg-ATP at 37°C for 600–900 s. S3 cleavage was monitored as an increase in fluorescence at an excitation and emission of 320 nm and 420 nm, respectively (three or more replicates). Error bars represent SEM.
(B) StAR (5.6 μM) or TFAM (5 μM) were combined in buffer with Mg-ATP, and reactions were initiated by the addition of Lon (500 nM) at 37°C. Aliquots were removed at time points, reactions were terminated with 5× reducing sample buffer, and aliquots were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. ImageJ was used for determining the percent StAR and TFAM degraded after a 60 min incubation period with the same Lon variant (no replicates).
(C and D) Overexpression of (C) wild-type Lon-V5 and (D) p.Arg721Gly-V5 (green = V5 immunofluorescence) in ARPE-19 cells counterstained with Mitotracker Red (red) and DAPI (blue). Nontransfected cells display only red Mitotracker fluorescence.
(E) Coimmunoprecipitation (IP) of Lon-V5 by Lon-FLAG (co-overexpressed in HEK293T cell lysates) with anti-FLAG M2 antibody. Immunoblotting (IB) with anti-FLAG and V5 antibodies followed.
