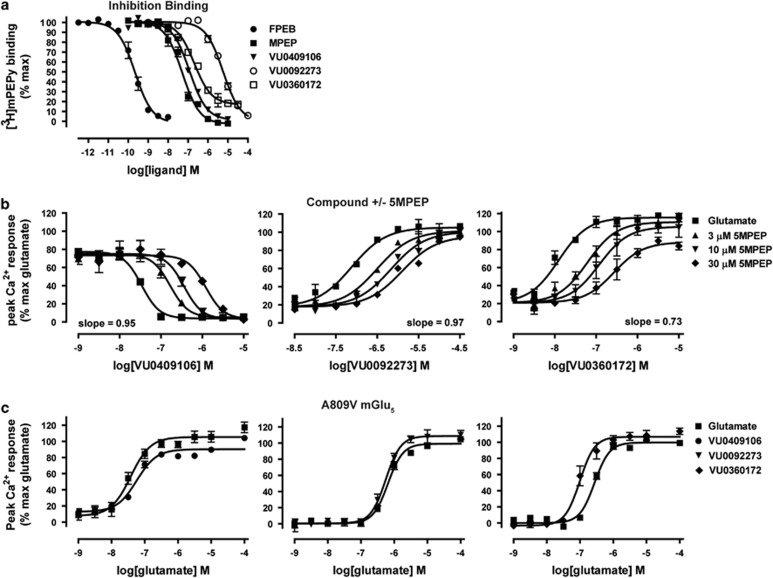
Figure 5.
VU0092273 and VU0409106 display differential interactions than VU0360172 with the MPEP (methyl-6-(phenylethynyl)pyridine hydrochloride) binding site of mGlu5 (metabotropic glutamate receptor subtype 5). (a) VU0092273 and VU0409106, but not VU0360172, fully inhibit [3H]methoxy-PEPy binding with potencies of 2.7 μM and 118 nM in mGlu5 rat cell membranes. (b) Addition of increasing concentrations of the neutral ligand 5MPEP (5-methyl-2-phenylethynyl-pyridine) results in a parallel rightward shift in VU0092273 and VU0409106 concentration–response curve with no change in maximum glutamate response. However, 5MPEP decreases in the maximum glutamate response evoked by VU0360172, and Schild regression analysis depicts a slope of 0.73, suggesting a noncompetitive interaction. (c) Introduction of the single-point mutation A809V, which abolishes activity at the mGlu5 allosteric MPEP-site, eliminated the shift in the glutamate concentration–response curves evoked by VU0092273 or VU0409106 observed in polyclonal wild-type rat mGlu5 cells, while only partially attenuating the VU0360172-induced fold shift (4.4 to 2.8). Data represent the mean±SEM of three independent experiments performed in duplicate.