Abstract
Background
Extragastrointestinal stromal tumors (EGISTs) are extremely rare mesenchymal tumors histologically and immunophenotypically similar to GI stromal tumors (GISTs). The aim of this study was to analyze the clinicopathological factors and treatment outcome in 13 patients with EGISTs treated at a tertiary care center.
Methods
Of 109 patients with GISTs treated at our center between April 2002 and December 2012, 13 patients with EGISTs were analyzed for clinicopathological factors and treatment outcome.
Results
Mean age was 45.8 (range 30-61) years, and females constituted 62% with a male:female ratio of 0.6:1. The most common tumor sites were mesentery in 10 patients and retroperitoneum in 3 patients. Mean tumor size was 11.7 (range 5-18) cm. Four (31%) patients were metastatic at presentation, the most common site of metastases being the liver in 3 (75%) patients. Lymph node enlargement was seen in 2 patients. Surgery was performed in 8 (62%) patients, 7 with localized disease, and 1 with metastatic disease. R0 resection was achieved in 3 (38%) patients. Five (71%) patients were considered as high-risk. Recurrences were seen in 3 patients (patient 3, 5 and 13) with localized disease after surgical resection, at 18, 7 and 137 months, respectively. At the last follow up, 7 patients were alive and 6 died of disease progression. The median overall survival was 34 (7-148) months.
Conclusions
EGISTs present at a younger age in the developing than in the developed countries. Females are more commonly affected than males. Lymph node metastases may be commonly present.
Keywords: Keywords Extrgastrointestinal stromal tumors, mesentery, retroperitoneum
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the GI tract which arise from the interstitial cells of Cajal [1]. The most common site of involvement of GIST is the stomach (60-70%) followed by the small intestine (20-30%), whereas the colon and rectum (5-12%) and esophagus (2-5%) are less commonly affected [2]. Reith et al described tumors histologically and immunophenotypically similar to GISTs, which occurred in the soft tissues of the abdomen, thus named as “extragastrointestinal stromal tumors (EGISTs).” EGISTs were found to have more aggressive course, comparable to small intestinal GISTs, than gastric GISTs. Most EGISTs express c-kit receptor on the basis of which it has been suggested that these tumors recapitulate the phenotype of the GI pacemaker cell (interstitial cell of Cajal) [3]. EGISTs account for <10% of the stromal tumors. Most EGISTs arise from omentum, mesentery, retroperitoneum, and other undefined abdominal sites [4].
Due to scarcity of data on EGISTs, we aimed to review our experience with 13 patients treated at our center and determine the clinical profile and treatment outcome.
Patients and methods
A total of 109 patients with GISTs were diagnosed and treated at our center, over a period of 10 years (from April 2002 to December 2012). Of 109 patients, 13 patients with EGISTs were analyzed for clinicopathological factors and treatment outcome.
Patient data included age, sex, presenting symptoms, primary sites, and sites of metastases. Presentation status was categorized as non-metastatic or metastatic. The tumor characteristics included size, mitotic count, morphology, and grade. The diagnosis was established on the basis of histopathological examination and immunohistochemistry. The immunohistochemical profile was performed using a panel of CD117, CD34, vimentin, desmin, S-100 and smooth-muscle actin (SMA). The histologic diagnosis of all tumors diagnosed outside our center was confirmed by members of the Pathology department. Mutation analysis for kit and platelet-derived growth factor receptor-α (PDGFRA) was not done in any patient. Metastatic workup was done with computed tomography (CT) scan of thorax, abdomen, and pelvis. Patients with localized disease were considered for surgical resections. The type of resection performed was classified as R0 if there was no residual disease; R1 when there was microscopic residual disease; and R2 when there was macroscopic residual disease. The risk of recurrence in patients with localized disease after surgery was evaluated using the Fletcher’s criteria based on tumor size and mitotic index [5].
Imatinib was given to all patients with advanced and metastatic disease and in adjuvant settings to all patients with high-risk disease. Imatinib was given at a recommended dose of 400 mg/day. Response was assessed clinically and radiologically by contrast-enhanced CT of the primary and metastatic site. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were defined according to response evaluation criteria in solid tumors. Dose escalation of imatinib (600 mg/day or 800 mg/day) or second-line therapy with sunitinib was considered in patients who showed progression on imatinib. Patients who did not tolerate the standard doses received lower doses of imatinib. All events were calculated from the first registration date to our institute to the last day of follow up or death from any cause.
Results
The clinicopathological factors and treatment outcome of patients is listed in Table 1. Mean age was 45.8 (range 30-61) years. Males and females constituted 38% and 62%, respectively, with a male:female ratio of 0.6:1. The most common presenting symptom was abdominal pain in 9 (69%), followed by a palpable abdominal lump in 5 (38%) patients. Four (31%) patients were metastatic at baseline, the most common site of metastases being liver in 3 (75%) patients. Abdominal lymph node enlargement was seen in 2 patients with liver metastases.
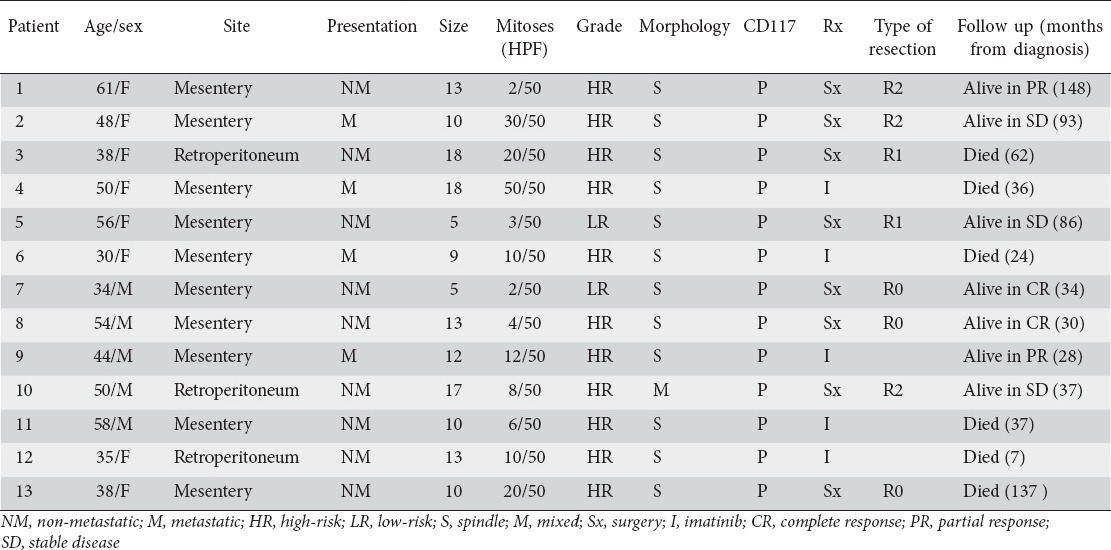
Table 1.
Extragastrointestinal stromal tumors: clinicopathologic factors and treatment outcome in 13 patients
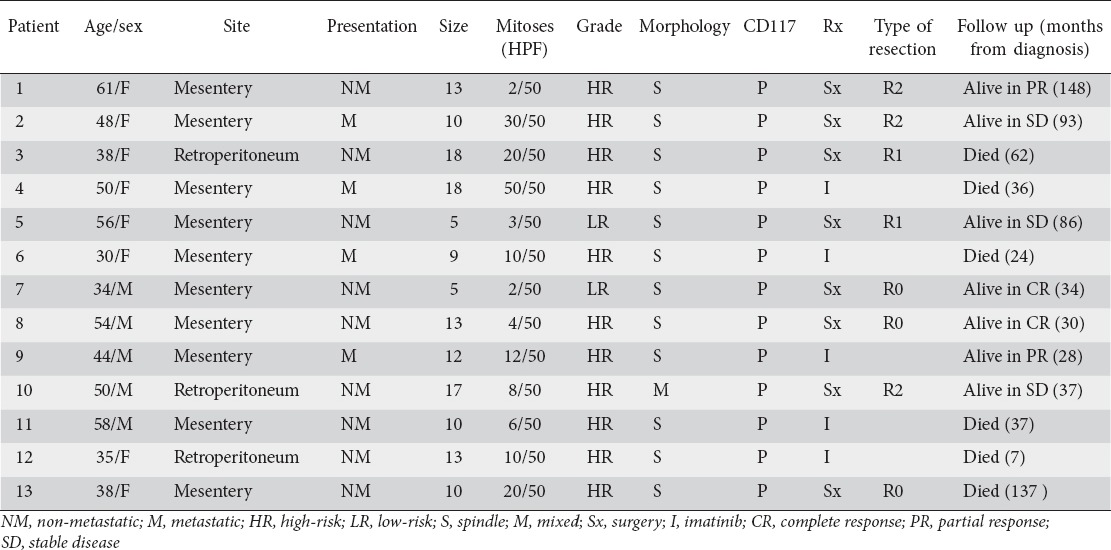
The most common primary sites of tumor were mesentery in 10 (77%) and retroperitoneum in 3 (23%) patients. Mean tumor size was 11.7 (range 5-18) cm. More than half (54%) of patients had tumors larger than 10 cm. Morphologically, 92% tumors were spindle cell and 8% were mixed spindle and epithelioid variety. Immunohistochemical analysis showed CD117 positivity in 13 (100%), CD34 in 5 (38%), SMA in 5 (38%), and S-100 in 3 (23%) patients.
Eight (62%) patients underwent surgical resection, 7 patients with primary disease and 1 patient with metastatic disease. R0 resection was achieved in 3 patients, R1 in 2 patients and R2 in 3 patients. Of the 7 patients who underwent surgical resection for localized disease, 5 (71%) were considered as high-risk. Adjuvant therapy with imatinib was given in all high-risk patients. Imatinib was used as a primary therapy in 8 patients (5 patients with unresectable disease and 3 patients with R2 resection). The responses seen with imatinib were: PR in 3 patients; SD in 2 patients; and PD in 3 patients. Neoadjuvant therapy with imatinib was not given in any patient. Imatinib was well tolerated by all but one patient who required dose reduction to 300 mg/day because of recurrent dyspeptic symptoms.
Recurrences were seen in 3 patients with localized disease (patients 3, 5, and 13) at 18, 7, and 137 months respectively after surgical resection, 2 (patients 3 and 13) had local recurrence and 1 (patient 5) developed liver metastases. The dose of imatinib was increased to 600 mg/day in 3 patients (patients 3, 5, and 13) with recurrent disease, and in 2 patients (patients 6 and 12) with PD, whereas sunitinib was used in 1 patient (patient 11) with PD who was intolerant to imatinib. SD was achieved in 2 patients (patients 3 and 5) with imatinib dose escalation, whereas the rest of the patients had PD.
Follow up was available for all patients. At last follow up, 7 patients were alive (2 in CR and 5 with disease) and 6 patients died of the disease. The median overall survival was 34 (7-148) months.
The limitation of this study was lack of mutation testing for Kit or PDGFRA.
Discussion
EGISTs arise exclusively outside the GI tract and display a similar range in histologic appearance and immunophenotypic profile as GISTs. They arise most commonly in the mesentery, omentum, and retroperitoneum [3]. However, cases of EGISTs have been reported in the pancreas, liver, gallbladder, urinary bladder, vagina, pelvic cavity, pleura, prostate, mesoappendix, seminal vesicle, and as vulvovaginal or rectovaginal masses [6-16]. In our study, EGISTs were located mainly in mesentry [10] and retroperitoneum [3].
In the largest study of 48 patients with EGISTs, mean age was 58 (range 31-82) years. However, in our study, the mean age was 45.8 years which is a decade earlier than already reported in literature [3]. EGISTs are reported to be more common in females than males [3,17,18]. We also found the female predominance in our study.
EGISTs commonly present as enlarging abdominal masses of variable duration often accompanied by vague abdominal pain. The tumors vary in size from 2.1 to 32 cm, most of them being greater than 10 cm [3,19]. The most common presenting symptom in our study was abdominal pain in the majority of the patients, followed by an abdominal lump in some. Mean tumor size was 11.7 cm. On histological examination, most of the tumors were spindle cell; epithelioid pattern was not found in any of the tumors. This finding differs from the study by Reith et al where the majority of tumors were of epithelioid type [3]. Immunohistochemical characteristics of EGISTs are similar to GISTs with the majority of patients having positivity for CD117 (Kit receptor) (100%) and CD34 (50-80%) [3,13]. Likewise, in our study, CD117 positivity was seen in all the patients, however, CD34 positivity was found in only 38% patients, lower than the previous reports. Mutation analysis for kit and PDGFRA was not done in our study. Lymph node enlargement, not described in any other study, was found in 2 patients.
There is limited data with regard to survival and prognostic factors of EGISTs. In a study by Reith et al, 12 of 31 (39%) patients with EGIST developed metastases or died of tumor at a median follow up of 24 months. The factors associated with adverse outcome were cellularity, mitotic activity (>2/50 high-power fields), and necrosis. Tumor size was not found to affect the survival [3]. Barros et al reported an average overall survival of 26.4 months in their study of 9 patients [18]. Mesenteric EGISTs are considered to have an unfavorable outcome compared to omental EGISTs [20,21].
The median overall survival in our study was 34 (7-148) months. Three (43%) patients with localized disease developed recurrence after surgical resection. Six (46%) patients died of disease. None of the factors could predict survival in our study, which may be explained by the small number of patients. Table 2 shows comparison of clinicopathological factors of our study with other published literature on EGIST.
Table 2.
Comparison of clinicopathological factors with other studies
In conclusion, EGISTs present a decade earlier in developing countries than West. These are seen more commonly in females. Lymph node metastases may be present which needs to confirmed by histopathology. The limitation of our study was lack of mutation analysis.
Summary Box.
What is already known:
Extragastrointestinal stromal tumors (EGISTs) constitute 5-7% of stromal tumors, affect more frequently female patients >55-60 years old, and have an aggressive course
What the new findings are:
EGISTs may actually be more frequent, as they were found at a rate of 12% of all stromal tumors with female predominance
Our patients were diagnosed with EGIST at an age of 10 years younger than the patients from previous reports
Lymph node enlargement, not reported in any other study and not seen in GIST, may be present in EGIST
EGIST course may not be that aggressive
Larger studies with longer follow up are warranted to investigate survival
Biography
All India Institute of Medical Sciences; Indraprastha Apollo Hospital, New Delhi, India
Footnotes
Conflict of Interest: None
References
- 1.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT):gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors – Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 3.Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 4.Dubey U, Rumpa D, Agrawal A, Pantola C, Verma N. Malignant extragastrointestinal stromal tumours: what are the prognostic features to depend upon? J Clin Diagn Res. 2011;52:369–371. [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 6.Vij M, Agrawal V, Pandey R. Malignant extra-gastrointestinal stromal tumor of the pancreas. A case report and review of literature. JOP. 2011;12:200–204. [PubMed] [Google Scholar]
- 7.Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606–1608. doi: 10.5858/2003-127-1606-PMGSTO. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol. 2000;24:1420–1423. doi: 10.1097/00000478-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Krokowski M, Jocham D, Choi H, Feller AC, Horny HP. Malignant extragastrointestinal stromal tumor of bladder. J Urol. 2003;169:1790–1791. doi: 10.1097/01.ju.0000062606.13148.04. [DOI] [PubMed] [Google Scholar]
- 10.Weppler EH, Gaertner EM. Malignant extragastrointestinal stromal tumor presenting as a vaginal mass: report of an unusual case with literature review. Int J Gynecol Cancer. 2005;15:1169–1172. doi: 10.1111/j.1525-1438.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirano H, Yoshida T, Yoshimura H, et al. Extra-gastrointestinal stromal tumor of the pelvic cavity: case report. Med Mol Morphol. 2012;45:173–177. doi: 10.1007/s00795-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 12.Yi JH, Sim J, Park BB, et al. The primary extra-gastrointestinal stromal tumor of pleura: a case report and a literature review. J pn J Clin Oncol. 2013;43:1269–1272. doi: 10.1093/jjco/hyt158. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZH, Feng GW, Liu ZF, et al. Ayoung man with primary prostatic extra-gastrointestinal stromal tumor: a rare case report and review of the literature. Int J Clin Exp Pathol. 2014;7:1764–1770. [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Cui Y, Ren G, Wang J, Wu X. Extragastrointestinal stromal tumor of the mesoappendix: CT findings and a review of the literature. World J Surg Oncol. 2012;10:211. doi: 10.1186/1477-7819-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou Y, Wang Y, Xu R, Li D, Zhao X. An extragastrointestinal stromal tumor originating from the seminal vesicles: a case report and review of the literature. Oncol Lett. 2013;6:947–949. doi: 10.3892/ol.2013.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam MM, Corless CL, Goldblum JR, Heinrich MC, Downs-Kelly E, Rubin BP. Extragastrointestinal stromal tumors presenting as vulvovaginal/rectovaginal septal masses: a diagnostic pitfall. Int J Gynecol Pathol. 2006;25:288–292. doi: 10.1097/01.pgp.0000215291.22867.18. [DOI] [PubMed] [Google Scholar]
- 17.Patnayak R, Jena A, Parthasarathy S, et al. Primary extragastrointestinal stromal tumors: a clinicopathological and immunohistochemical study: a tertiary care center experience. Indian J Cancer. 2013;50:41–45. doi: 10.4103/0019-509X.112298. [DOI] [PubMed] [Google Scholar]
- 18.Barros A, Linhares E, Valadão M, et al. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepatogastroenterology. 2011;58:865–868. [PubMed] [Google Scholar]
- 19.Weiss SW, Goldblum JR. Extragastrointestinal stromal tumor. In: Enzinger FM, Weiss SW, editors. Soft Tissue Tumors. 5th ed. St. Louis: Mosby; 2008. pp. 565–579. [Google Scholar]
- 20.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Llenas-García J, Guerra-Vales JM, Moreno A, et al. Primary extragastrointestinal stromal tumors in the omentum and mesentery: a clinicopathological and immunohistochemical study. Hepatogastroenterology. 2008;55:1002–1005. [PubMed] [Google Scholar]


