Abstract
Achalasia is a primary neurodegenerative disorder of the esophagus characterized by loss of function of the lower esophageal sphincter (LES) and of esophageal peristalsis, which causes symptoms such as dysphagia, regurgitation, weight loss, and chest pain. Esophageal manometry is the gold standard for the diagnosis of achalasia. The typical manometric features are incomplete relaxation of a frequently hypertensive LES and lack of peristalsis in the tubular esophagus. High-resolution manometry using catheters with 36 solid-state sensors spaced 1cm apart has more and more replaced water-perfused and pull-through manometry. However, the main innovation of this method is the conversion of pressure data into a topographical plot. The data can be modified using interpolation to generate high-resolution esophageal pressure topography (HREPT). HREPT is more sensitive, provides more detailed information, and is easier to perform than conventional manometry. Introduction of HREPT had an impact especially on the diagnosis and management of achalasia. A clinically relevant impact was achieved by the identification of 3 clinical subtypes which seem to predict treatment outcomes. This review analyzes the progress made in the diagnosis and management of achalasia since the recent introduction of HREPT.
Keywords: Keywords Achalasia, high-resolution manometry, esophageal pressure topography, Chicago classification
Introduction
Idiopathic achalasia is a rare primary esophageal motor disorder of unknown etiology, with an estimated incidence of 1 case per 100,000 of the general population [1]. It represents a neurodegenerative disorder, in which neurons of the myenteric plexus are destroyed. Therefore achalasia is characterized by loss of function of the lower esophageal sphincter (LES) and esophageal peristalsis, which causes symptoms such as dysphagia, regurgitation, weight loss, and chest pain.
The diagnosis of achalasia is suspected clinically on the basis of the symptoms mentioned above and confirmed by diagnostic tests. Upper gastrointestinal endoscopy and barium swallow are indicative of achalasia and esophageal manometry is the gold standard for its diagnosis. The typical features are incomplete relaxation of a frequently hypertensive LES and a lack of peristalsis in the tubular esophagus. High-resolution manometry (HRM) is more sensitive, provides more detailed information, and is easier to perform than conventional manometry (CM) [2-4].
HRM generates a detailed pressure topography of the esophagus which identifies compartmental pressurization in the distal esophagus and has recently been renamed to high-resolution esophageal pressure topography (HREPT) [2,5,6]. This pressure topography plotting has been used to create a new classification of idiopathic achalasia of 3 different subtypes with possible clinical implications [7].
This review provides an evidence-based approach of the progress made in the diagnosis and treatment of achalasia since the introduction of HREPT.
Esophageal manometry
The procedural basis for both types of manometry, i.e. CM or HRM is the same. Both begin with placement of the manometry catheter transnasally until the distal pressure sensors cross the esophagogastric junction (EGJ) and enter the stomach. Patients are instructed to fast overnight and to omit any medications that might affect motility for 48 h prior to the manometry.
CM
CM can be performed with the use of a low-compliance capillary perfusion system or a solid state assembly with pressure sensors, usually spaced at 3-5 cm intervals. A stationary pull-through method is used to determine the position of the LES and to identify the pressure inversion point and a high-pressure zone. The LES resting pressure and relaxation in response to 5 wet swallows is measured with the pressure sensor in the middle of the LES high-pressure zone. Esophageal body motility is assessed by repositioning of the pressure sensors into the body. The response to 10 wet swallows separated by an interval of at least 30 sec is tested.
The diagnosis of classic achalasia is characterized by complete absence of peristalsis in the body of the esophagus (simultaneous contractions with amplitudes <40 mmHg or no apparent esophageal contraction) and incomplete relaxation of a hypertonic or normotonic LES. Atypical presentations of achalasia have been described by conventional manometry. These include cases with preserved peristalsis and/or esophageal contractions with amplitudes greater than 40 mmHg, the latter situation often being referred to as “vigorous achalasia” [8-10].
HRM
An HRM device is composed of multiple, closely-spaced pressure sensors (usually 1 cm apart). HRM provides much more information than CM, as data are not lost in the 3-5 cm-sized gaps between the sensors of CM [11].
In contrast to the station pull-through method of the CM, the HR-assembly needs no further repositioning once it has been placed across the EGJ. This makes the procedure much more comfortable for the patients. Advantages of HRM over CM is the simultaneous assessment of the upper and LES as well as the esophageal body peristalsis with a single series of swallows, which makes the data acquisition period shorter than with CM.
However, the main innovation of this method is the conversion of pressure data into a topographical plot. The data can be modified using interpolation to generate HREPT plots that are color-coded, spatiotemporal representations of pressure recordings in the esophagus (Clouse Plots). Colors are assigned to the pressures with cool colors (blue and green) for lower pressures and warm colors (red and yellow) for high pressures [12-14].
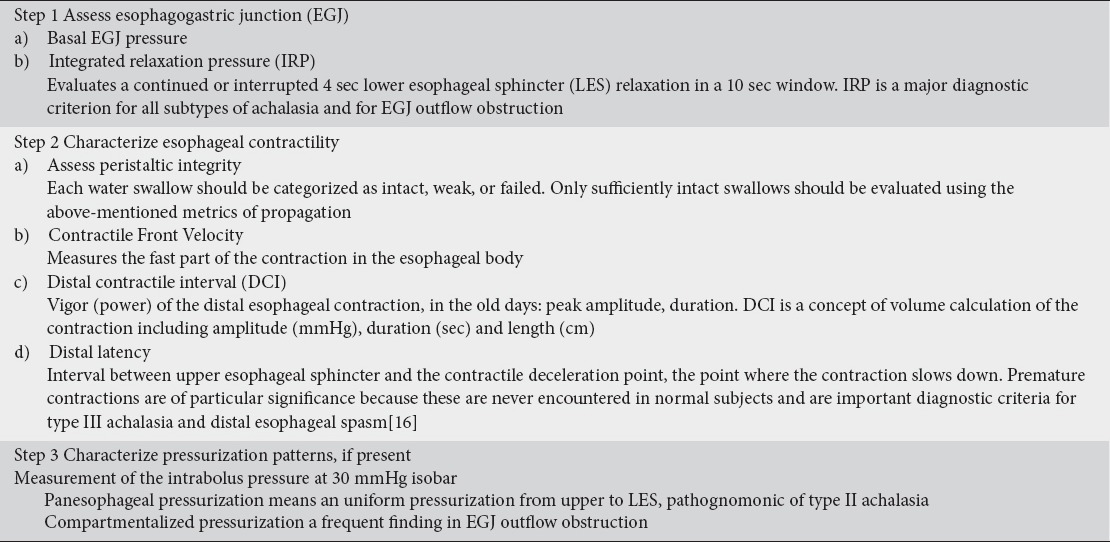
Analysis of a HREPT study is performed by using a stepwise approach focused on an algorithm-based scheme that first assesses EGJ relaxation pressures and subsequently uses individual swallow patterns defined by HREPT metrics to further subclassify the patient into specific categories [12]. A stepwise approach to esophageal pressure topography is shown in Table 1 [15].
Table 1.
Steps of analyzing high-resolution esophageal pressure topography studies
Impact on the diagnosis of achalasia
General improvements
The implementation of HREPT involved the establishment of a structured classification system, the so-called Chicago classification of esophageal motility. After all of the swallows are analyzed with the stepwise approach described above (Table 1) the data are used in the Chicago classification to make a diagnosis. As shown in Fig. 1 at first the LES function is assessed and then esophageal motor function is characterized. A key feature of the Chicago classification [5] is the hierarchical categorization of esophageal motility disorders, with 4 general groupings; achalasia, esophageal outlet obstruction, abnormalities of esophageal motor function not seen in asymptomatic controls [such as absent peristalsis, distal esophageal spasm, and hypercontractile (jackhammer) esophagus], and borderline abnormalities of esophageal function commonly found in asymptomatic individuals [e.g. weak or hypertensive (nutcracker esophagus) peristalsis].
Figure 1.
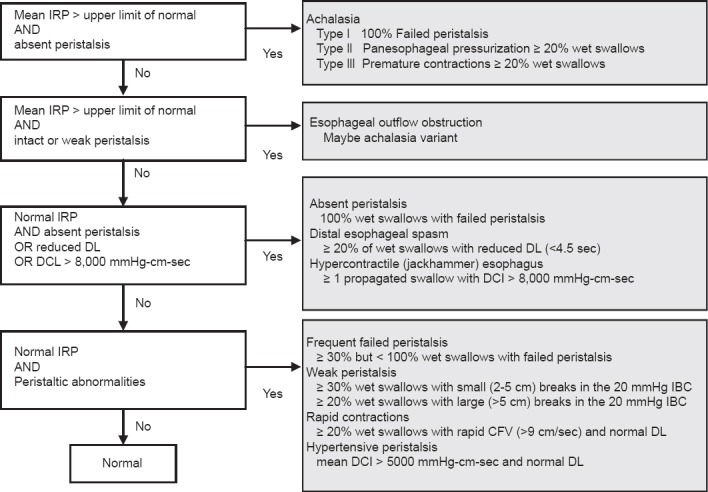
The Chicago classification algorithm. Adapted from Conklin 2013 [13], Bredenoord 2012 [22], Carlson and Pandolfino 2013 [12]
IRP, integrated relaxation pressure; DCI, distal contractile integral; DL, distal latency; CFV, contraction front velocity; IBC, isobaric contour
One of the cardinal criteria for the diagnosis of achalasia is impaired LES (or EGJ) relaxation, although no universally accepted criteria were ever available for defining impaired LES relaxation by CM. HREPT can measure LES pressure changes more accurately than CM [16,17], because of the implementation of the integrated relaxation pressure, the average minimum EGJ pressure for 4 sec of relaxation within 10 sec of swallowing (upper sphincter relaxation). This is an important improvement because LES relaxation with swallowing rarely reaches the level of the intragastric pressure and, when it does, it is only for a brief interval. Therefore, it might be difficult in CM to distinguish artefacts from real impaired swallow induced relaxation. Furthermore, the LES moves proximally at an average of 2 cm during swallowing as a consequence of longitudinal muscle contraction and esophageal shortening and can falsely be diagnosed as sphincter relaxation during CM. This problem of ‘pseudorelaxation’ limits the specificity of CM for the diagnosis of achalasia because falsely diagnosed relaxation of the LES could change the manometric diagnosis from achalasia to absent peristalsis, or from spastic achalasia to diffuse esophageal spasm [11]. The other cardinal feature for the diagnosis of achalasia is absent peristalsis. However, absent peristalsis is not synonymous with absent pressurization within the tubular esophagus, difficult to measure with CM. With HREPT it is possible to show esophageal pressurization as elevated intrabolus pressure and thus has emerged as one new criterion that might help distinguish certain subtypes of the disease [11].
It seems that these new diagnostic tools improve the sensitivity and specificity of HREPT over CM to diagnose achalasia and lead to possibilities of early and guided intervention [18]. Besides the higher sensitivity in the diagnosis of LES relaxation/impairment, it became apparent that many esophageal motor disturbances could be recognized as distinct patterns [19]. These attributes have led to improved inter-observer agreement among interpreters and provide a more user-friendly method for teaching trainees [20,21].
Specific achalasia subtypes
The classification of achalasia has evolved with the introduction of HREPT. Different achalasia subtypes have been described, all of which are associated with abnormal EGJ relaxation and are categorized based on the pattern of esophageal body contraction and pressurization (Table 2, Fig. 2) [7,22]. Thus, by HREPT, achalasia can be subtyped into classic achalasia (with no esophageal pressurization by swallowing), achalasia with esophageal pressurization (characterized by compression of the water bolus between the upper and LES), and spastic achalasia (characterized by nonpropulsive, high-amplitude contractions in the esophageal body) [11]. The impact of this new classification system on the clinical management of achalasia will be described below.
Table 2.
Manometric variants of achalasia

Figure 2.
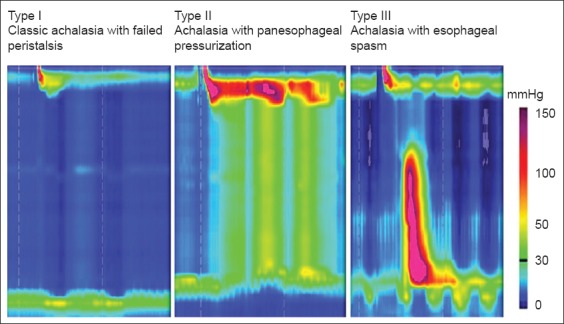
High-resolution esophageal pressure topography showing the 3 different types of achalasia. Courtesy of Niebisch, Mainz, Germany
Differential diagnosis of abnormal LES relaxation
HREPT studies demonstrated a population of patients with impaired LES relaxation with remaining peristaltic of the tubular esophagus that fails to meet diagnostic criteria of achalasia, similar to those with ‘atypical disorders of LES relaxation’ in CM [12]. This disorder was called functional obstruction in the first Chicago classification schemes. Further on, it could be demonstrated that these patients had an elevated intrabolus pressure, similar to patients with a known mechanical obstruction, and therefore it is now categorized as EGJ outflow obstruction (as shown in Fig. 2) [12,22]. However, because the primary dysfunction in EGJ outflow obstruction and achalasia is failure of swallow-induced LES relaxation, symptoms and treatment are often similar. It has been reported to occur in benign and malignant infiltrative disorders or may be a variant or earlier form of achalasia [23].
Impact on the treatment of achalasia
Since the underlying defect cannot be reversed, treatment of achalasia remains palliative. Therefore, the aim of all current therapies is the improvement in esophageal food passage by reducing the distal esophageal obstruction. Such improvement will lead to symptomatic relief of dysphagia, regurgitation, as well as weight gain.
This goal can be achieved by pharmacologic therapy; endoscopic treatment with pneumatic dilation (PD) or botulinum toxin (BOTOX) injection; or surgery. Recently, new therapeutic options such as stent implantation or peroral endoscopic myotomy have been reported [24,25]. However, the efficacy of these treatment options varies and the recommendation for the best therapy is still controversial. Although PD and Heller myotomy seem to be the most effective treatments for achalasia [26], until now the choice of treatment modality primarily depended on multiple factors, such as patients’ characteristics, clinical presentation, local expertise, and patient’s preference [27]. In the past, CM criteria for high recurrence rates after PD have been defined. These include younger age, male gender, and LES >10 mmHg post-dilation. However, some patients do not have a good response either to endoscopic or to operative approaches, whereas others remain symptom-free for more than 10 years after a single PD. Therefore other factors might play a role in the therapeutic success.
The new HREPT subclassification of achalasia distinguishes separate clinical phenotypes that might help predict response to therapy. This scheme is supported by 5 retrospective studies. Table 3 shows a summary of the published studies showing different treatment responses in patients with the 3 achalasia subtypes [4,18,28-30].
Table 3.
Summary studies demonstrating the different treatment results based on the achalasia subclassification
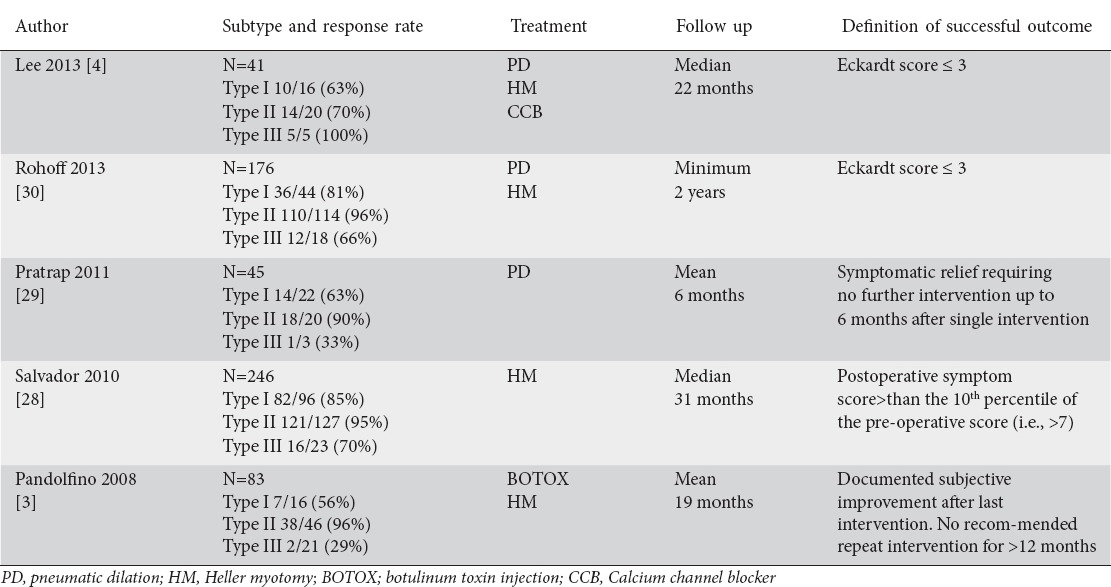
The study by Pandolfino et al [18] inaugurated the 3 subtypes of achalasia based on HREPT results and linked them to clinical outcomes. The authors found that type II patients were significantly most likely to respond to any therapy, such as BOTOX [71%], PD [91%], or Heller myotomy [100%]. In contrast, treatment response was lower for type I (56% overall) or type III (29% overall) patients. Logistic regression analysis found type II to be a predictor of positive treatment response, whereas type III predicted the poorest treatment response to all types of therapy.
Similarly, Salvador et al [28] analyzed 246 patients with different subtypes of achalasia (14.6% type I, 4.7% type II, and 30.4 % type III), all of whom were treated surgically with laparoscopic Heller-Dor myotomy. They were able to show that the best clinical outcome was achieved in patients with type II achalasia.
Pratap et al [29] analyzed 51 patients with HREPT, most of whom were treated with PD, and similarly demonstrated that patients with type II had the best response to PD compared with types I and III.
The esophageal pretreatment manometry data collected from 176 patients who participated in the European achalasia trial, in which patients with newly diagnosed achalasia were randomly assigned to PD or laparoscopic Heller-myotomy (LHM) with Dor’s fundoplication were examined by Rohoff et al [30]. These data underlined that a higher percentage of patients with type II achalasia were treated successfully with PD or LHM than patients with types I and III achalasia. The success rates in type II achalasia were high for both treatment groups but significantly higher in the PD group (100% vs. 93%). For type I achalasia, PD and LHM had similar rates of success (81% vs. 85%), whereas in type III achalasia LHM had a higher success rate than PD (86% vs. 40%).
Lee et al [4] studied 50 patients with achalasia diagnosed by CM and HREPT, 41 of whom received treatment. Treatment responses of PD and Heller’s myotomy in type I group were 71.4 and 50.0%, and in type II group 85.7 and 75.0%, respectively. In addition, all patients in type III group (n=5) showed good response to medical therapy. Type III patient have poor response rates to other treatment options and although medical treatment is generally not very effective in achalasia, it might lead to short-term symptomatic improvement in the related spasm [4]. Medical treatment is additionally prone to side effects, such as peripheral edema, headaches or hypotension [27]. Therefore, the “good response” shown in a very small number of patients needs to be validated by others prior to making firm recommendations. Currently, use of medical treatment is mostly limited to symptomatic relief in patients who have very early disease, or as a temporary measure.
These studies have methodological differences, according to the type of esophageal manometry, treatment modalities, definition of response, and time of follow up. Nevertheless, consistent across all studies was the observation that patients with type II achalasia have the best and patients with type III achalasia have the worst response to treatment. It seems that type II had the best results for PD and surgery seems benefit more type I achalasia patients. Although PD and surgery are not very effective in patients with type III achalasia medical therapy (BOTOX) might be an alternative for these patients.
These results suggest that achalasia subtypes represent unique clinical phenotypes and may offer criteria to plan the optimal treatment for the patient with achalasia, although more prospective data are needed to confirm these first results [31].
Impact on the pathophysiologic understanding
Another question which arises from these results is whether there are pathophysiological differences that can explain these different phenotypes. A study that used the combination of HREPT, multiple intraluminal impedance, and intraluminal ultrasonography of the esophagus demonstrated that longitudinal muscle contraction patterns are quite different in the 3 different types of achalasia. Type I achalasia showed minimal or no longitudinal muscle contraction of the esophagus. In type II achalasia, strong longitudinal muscle contraction was found, assumed to cause pan-esophageal pressurization; and in type III achalasia, both circular and longitudinal muscles contracted but there was severe discoordination between the 2 muscle layers.
Furthermore, it was shown that emptying occurred intermittently during periods of pan-esophageal pressurization in patients with type II achalasia whereas patients with achalasia of types I and III had no emptying or relatively normal emptying during most swallows, respectively [32].
It is still under discussion if the 3 different achalasia types represent different phenotypes during progression of the disease caused by the same process or if they represent 3 different pathophysiological defects [32].
The above-mentioned data and histopathological examinations support the latter assumption. Goldblum et al [33] assumed that vigorous achalasia might represent an early stage of the disease, because myenteric inflammation was seen, but the normal number of ganglion cells was not reduced. In contrast, in cases of typical achalasia complete aganglionosis can frequently be found and therefore this was regarded to be a late stage. However, none of the existing data propose propagation from type III to type I or II achalasia and therefore it is assumed that the underlying immune response might differ. In type III achalasia, a less intensive immune reaction could lead to neuronal dysfunction, lacking apoptosis. This subtype is often associated with spastic contractility, which could be mediated by cytokine-induced alterations rather than cell destruction [34]. In contrast, some patients with a progressive plexopathy might progress from a clinical picture with some preserved peristalsis or pressurization (similar to a type II phenotype) to end-stage disease with complete aperistalsis [35]. These variations in the progression of the disease and presentation of different phenotypes are likely a result of a varying intensity of the cytotoxic T-cell assault on the myenteric plexus [36].
Concluding remarks
HREPT is more sensitive, provides more detailed information, and is easier to perform than CM. Standardization is improved as a result of analysis algorithms, known as the Chicago classification, which has increased the early recognition of achalasia. A clinically relevant impact was achieved by the identification the 3 clinical subtypes of achalasia to predict treatment and disease outcome. Furthermore, implementation of the different phenotypes of achalasia increased our pathophysiologic understanding of the disease. This knowledge has the prospect to lead to an individualized management of the disease and improve outcomes for patients with achalasia.
Biography
DKD Helios Clinic, Wiesbaden, Germany
Footnotes
Conflict of Interest: None
References
- 1.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–374. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Kim N, Kim SE, et al. Clinical characteristics and treatment outcomes of 3 subtypes of achalasia according to the chicago classification in a tertiary institute in Korea. J Neurogastroenterol Motil. 2013;19:485–494. doi: 10.5056/jnm.2013.19.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago classification. J Clin Gastroenterol. 2008;42:627–635. doi: 10.1097/MCG.0b013e31815ea291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135:756–769. doi: 10.1053/j.gastro.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S, Lipshutz W. Lower esophageal sphincter dysfunction in achalasia. Gastroenterology. 1971;61:814–820. [PubMed] [Google Scholar]
- 9.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freidin N, Traube M, Mittal RK, McCallum RW. The hypertensive lower esophageal sphincter. Manometric and clinical aspects. Dig Dis Sci. 1989;34:1063–1066. doi: 10.1007/BF01536375. [DOI] [PubMed] [Google Scholar]
- 11.Bansal A, Kahrilas PJ. Has high-resolution manometry changed the approach to esophageal motility disorders? Curr Opin Gastroenterol. 2010;26:344–351. doi: 10.1097/MOG.0b013e32833aaf61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson DA, Pandolfino JE. High resolution manometry and esophageal pressure topography: filling the gaps of conventional manometry. Gastroenterol Clin North Am. 2013;42:1–15. doi: 10.1016/j.gtc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conklin JL. Evaluation of esophageal motor function with high-resolution manometry. J Neurogastroenterol Motil. 2013;19:281–294. doi: 10.5056/jnm.2013.19.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinicalesophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 15.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141:469–475. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high- resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol. 2006;290:G1033–G1040. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol. 2007;293:G878–G885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clouse RE, Prakash C. Topographic esophageal manometry: an emerging clinical and investigative approach. Dig Dis. 2000;18:64–74. doi: 10.1159/000016967. [DOI] [PubMed] [Google Scholar]
- 20.Grubel C, Hiscock R, Hebbard G. Value of spatiotemporal representation of manometric data. Clin Gastroenterol Hepatol. 2008;6:525–530. doi: 10.1016/j.cgh.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Soudagar AS, Sayuk GS, Gyawali CP. Learners favour high resolution oesophageal manometry with better diagnostic accuracy over conventional line tracings. Gut. 2012;61:798–803. doi: 10.1136/gutjnl-2011-301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24:57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–2225. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu YQ, Cheng YS, Tang GY, Li MH, Zhao JG, Li F. Comparison of temporary stent insertion with pneumatic dilation of the same diameter in the treatment of achalsia patients: a retrospective study. J Gastroenterol Hepatol. 2010;25:499–505. doi: 10.1111/j.1440-1746.2009.06107.x. [DOI] [PubMed] [Google Scholar]
- 25.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt AJ, Eckardt VF. Should pneumatic dilation be the primary treatment strategy? Nat Rev Gastroenterol Hepatol. 2010;7:188–190. doi: 10.1038/nrgastro.2010.33. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15:3969–3975. doi: 10.3748/wjg.15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvador R, Costantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg. 2010;14:1635–645. doi: 10.1007/s11605-010-1318-4. [DOI] [PubMed] [Google Scholar]
- 29.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144:718–725. doi: 10.1053/j.gastro.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Carlson DA, Pandolfino JE. The Chicago criteria for esophageal motility disorders: what has changed in the past 5 years? Curr Opin Gastroenterol. 2012;28:395–402. doi: 10.1097/MOG.0b013e3283530f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong SJ, Bhargava V, Jiang Y, Denboer D, Mittal RK. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139:102–111. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomytomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–654. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 34.Bruley des Varannes S, Chevalier J, Pimont S, et al. Serum from achalasia patients alters neurochemical coding in the myenteric plexus and nitric oxide mediated motor response in normal human fundus. Gut. 2006;55:319–326. doi: 10.1136/gut.2005.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicodème F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention to Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol. 2013;11:131–137. doi: 10.1016/j.cgh.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high resolution manometry. Gastroenterology. 2013;145:954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


