Abstract
BACKGROUND:
Currently, benefits of robotic surgery in patients with benign gynecological conditions remain unclear. In this study, we compared the surgical outcome of robotic and laparoscopic total hysterectomies and evaluated the feasibility of robotic surgery in cases with pelvic adhesions or large uterus.
MATERIALS AND METHODS:
A total of 216 patients receiving total hysterectomy via robotic or laparoscopic approach were included in this study. Of all 216 patients, 88 underwent robotic total hysterectomy and 128 underwent laparoscopic total hysterectomy. All cases were grouped by surgical type, adhesion score, and uterine weight to evaluate the interaction or individual effect to the surgical outcomes. The perioperative parameters, including operation time, blood loss, postoperative pain score, time to full diet resumption, length of hospital stay, conversion rate, and surgery-related complications were compared between the groups.
RESULTS:
Operation time and blood loss were affected by both surgical type and adhesion score. For cases with severe adhesions (adhesion score greater than 4), robotic surgery was associated with a shortened operation time (113.9 ± 38.4 min versus 164.3 ± 81.4 min, P = 0.007) and reduced blood loss (187.5 ± 148.7 mL versus 385.7 ± 482.6, P=0.044) compared with laparoscopy. Moreover, robotic group showed a lower postoperative pain score than laparoscopic group, as the effect was found to be independent of adhesion score or uterine weight. The grade-II complication rate was also found to be lower in the robotic group.
CONCLUSIONS:
Comparing to laparoscopic approach, robotic surgery is a feasible and potential alternative for performing total hysterectomy with severe adhesions.
Keywords: Pelvic adhesions, robotic surgery, total hysterectomy
INTRODUCTION
Pelvic adhesions are postinflammatory scar tissues between organs that occur after 70% to 90% of abdominal and pelvic surgeries.[1,2] Pelvic adhesions often occur following gynecological surgeries such as myomectomies, cystectomies, tubal surgeries, or rise from endometriosis or intra-abdominal infections. Extensive pelvic adhesions cause 74% of small bowel obstructions, 20% to 40% of infertility cases, and 19% of inadvertent enterotomies,[3] that result in morbidity and increased medical expenses. In the past, pelvic adhesions were considered a contraindication for laparoscopic surgeries. Severe adhesions increase surgical difficulties and operating times lead to conversion to laparotomy, and involved in 8.8% of re-admissions,[4] which is time consuming and increases medical costs.[5]
Since 2005, robotic surgery was approved by the US Food and Drug Administration (FDA) for gynecological procedures.[6] Several reports have demonstrated that gynecological robotic surgery is associated with less blood loss, lower operation time, shorter hospitalization, and a lower conversion rate to laparotomy in complicated or cancer surgeries.[7,8,9,10,11,12,13,14,15,16] However, robotic surgery provides greater short-term benefits to patients suffering from benign diseases with complicated surgical conditions remains largely unclear.[17,18,19] Moreover, the learning curve for robotic platform and the experience of the surgeons also affect the surgical performance of robotic procedures.[20,21] Wright et al. suggested that robotic surgery showed no significant clinical advantage than laparoscopy for treating benign gynecological conditions.[22] However, robotic surgery might still have a role in managing complicated surgical condition, such as severe pelvic adhesion or large pelvic mass. In our previous study, we have investigated the short-term surgical outcome of robotic surgery in managing stage IA to IIB cervical cancer.[23] In this study, we make further efforts to compare the surgical outcomes of robotic and laparoscopic total hysterectomies with pelvic adhesions or large uterine weight. The outcomes may help us to clarify the role of robotic surgery in managing complicated benign gynecological diseases.
MATERIALS AND METHODS
Between June 2011 and December 2013, 216 women were included in this study. All patients had their clinical diagnosis confirmed and received surgical treatment by a single surgeon at Taipei Medical University Hospital. Inclusion criteria were:
Age between 35 years and 55 years,
Body mass index between 16 and 35 kg/m2,
Received total hysterectomy, and
No concomitant procedures.
Exclusion criteria were:
A present or past history of cancer,
An absence of pathology confirmation, and
Incompletely recorded data.
By medical chart review, two cohorts of patients were included by surgical type: robotic group and laparoscopic group. To analyze the possible factors that affect the outcomes of each surgical type, patients were further subgrouped according to their adhesion severity and uterine weights in each cohort. To evaluate the impact of uterine weight to surgical outcomes, patients from the same population were grouped into large uterine weight group (uterine weight >300 g) or small uterine weight group (uterine weight <300 g), corresponding with previous reports regarding the management of large uterus.[24,25]
For the setting of laparoscopic surgery, the camera port was set at the umbilicus. The second and third ports were placed at 8-10 cm caudal-lateral to the scope at the left side of the patient and 8-10 cm caudad to the scope on the midline, respectively. The fourth assistant port was set at the right side of the patient, 8-10 cm caudal-lateral to the scope as needed. As to robotic surgery, the port sites were 6 cm above the umbilicus for the scope, and 8 to 10 cm caudal-lateral to the scope for the side arms at each side of the patient, respectively. For complicated cases that an accessory port was required, a fourth trocar was set at 6 to 8 cm caudal-lateral to the left arm. A survey of the operative field was performed before the main procedure. After locating the uterine artery and the ureter, bilateral ligation of the uterine arteries was performed as previously described,[26] followed by the main procedure. Total hysterectomy was then performed by robotic or laparoscopic approach, followed by transvaginal suturing to reduce the risk of vaginal dehiscence.[27] It allows surgeons to remove large uterus piece by piece via vagina, followed by the suturing of the cuff immediately. Another reason to suture the cuff transvaginally is that it saves the effort to maintain the pneumoperitoneum or to prevent the gas leak during suture procedure. For robotic surgery, it also saves the use of needle holder and lowers the cost of patients.
To evaluate the effect of adhesion severity to surgical outcomes, patients were grouped into none pelvic adhesion group (adhesion score equals to 0), mild adhesion group (with an adhesion score of 1 to 3), and severe adhesion group (with an adhesion score of 4 to 6). A scoring system from the Adhesion Scoring Group was adopted [Table 1].[28] The grading for each patient was assigned based on the surgical record and dictated by two surgeons blinded to the grouping of the cases. The adhesion severity was grading from 0 to 3 as: (0) none; (1) flimsy and avascular; (2) dense and/or vascular; or (3) cohesive. The extent of the adhesions (total adhesion area) was grading from 0 to 3 as: (0) none, (1) 1% to 25%, (2) 26% to 50%, or (3)>50%. The overall adhesion score for each patient represented the sum of the severity grading and extent grading.
Table 1.
Adhesion scoring system used for evaluating the severity and extent of pelvic adhesions

For each cohort, demographic data and perioperative parameters analyzed include age, body mass index (BMI), operation time, blood loss, laparotomy conversion, postoperative pain score, time to full-diet resumption, hospital stay, and surgical-related complications. The operation time (console time) was defined as the time from skin incision to close minus the docking time. The volume of blood loss was defined as the total volume of fluids collected by suction. The postoperative pain score were measured 24 h after the surgery by self-reported numerical rating scale (NRS-11), in which score 0 indicating no pain, and score 10 representing the worst pain imaginable. All patients received nonsteroidal anti-inflammatory drugs (NSAIDs) during postoperative care.
All patients were allowed to receive full diet after their first gas passing after surgery. To evaluate the time point of bowel function recovery for both groups, postoperative time to full diet resumption were also examined. The time to full diet resumption was defined as the number of postoperative days until the patients could tolerate regular intake of solid food. The length of hospital stay was defined as the number of postoperative days until the patient was discharged. The intraoperative and postoperative complications were abstracted from chart records and classified according to Clavien-Dindo classification, which stratifies complications into five grades according to their therapeutic and clinical effects.[29] For statistical analysis, all obtained data were analyzed using SPSS statistics (IBM, Armonk, NY, USA). The mean value and standard deviation (SD) of each perioperative parameter were reported. The statistical analysis was performed with 2-way ANOVA or chi-square analysis. A P value of less than .05 was considered statistically significant.
RESULTS
A total of 88 patients who received robotic total hysterectomy and 128 patients who received laparoscopic total hysterectomy were enrolled in this study. The patient characteristics were shown in Table 2. The age, body mass index (BMI), rates of prior pelvic surgeries, and indication for surgical intervention were listed. In the robotic group, 29.5% of patients had a history of prior pelvic surgeries; in the laparoscopic group, 25.0% of patients had prior pelvic surgeries. No significant difference was found between the groups, indicating that the study population from each surgical group was comparable.
Table 2.
Baseline characteristics of the enrolled patients
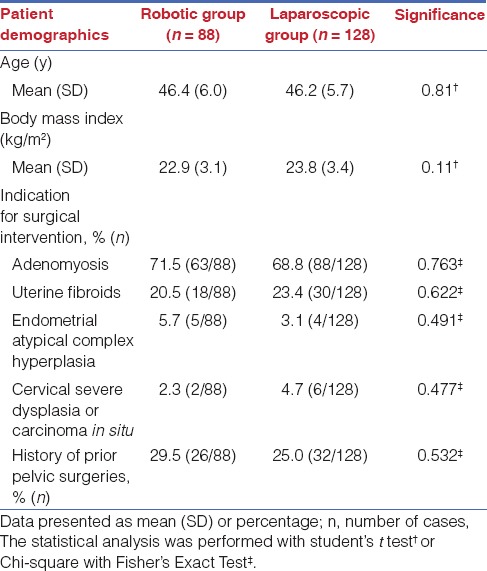
To evaluate the association of pelvic adhesions to the short term surgical outcomes by different surgical approaches, we grouped the patients by adhesion score and evaluated the peri-operative parameters in Table 3. Two-way ANOVA was applied to examine the interaction effects of covariables (surgical type and adhesion score) to each peri-operative parameters. The interactions of surgical type and adhesion score were significant for operation time (P < 0.001) and blood loss (P < 0.001), indicating that adhesion score and surgical type both contribute to the significant differences between groups. Compare to laparoscopy, robotic surgery was associated with a shortened operation time (113.9 ± 38.4 min versus 164.3 ± 81.4 min, P=0.007) in severe adhesion group (score>4). Blood loss was also found to be reduced in the robotic groups as compared to the laparoscopic group (187.5 ± 148.7 mL vs 385.7 ± 482.6, P=0.044) in severe adhesion group. The interactions of surgical type and adhesion score were not significance for 24-h pain score (P=0.432), time to full diet resumption (P=0.090), and hospital stay (P=0.429). However, the main effect of surgical type for 24-h pain score was significant (0.048), indicating that the effect of surgical type, but not adhesion score, contributes to the significant differences between groups. The result indicated that robotic surgery was associated with lower 24-h postoperative pain, no matter the adhesion score of the cases. Moreover, the time to full diet resumption was found to be comparable between robotic group and laparoscopic group, indicating that the time for bowel function recovery in both group were similar. All patients tolerated solid full diet less than 1.5 days postoperatively, and no interaction effect was found for surgical type and adhesion score to the outcome. The length of hospital stay was also shown to be comparable between the groups, whereas all patients were discharged less than 6 days postsurgery. No interaction effect was found for surgical type and adhesion score to the outcome.
Table 3.
Peri-operative parameters by severity of pelvic adhesions
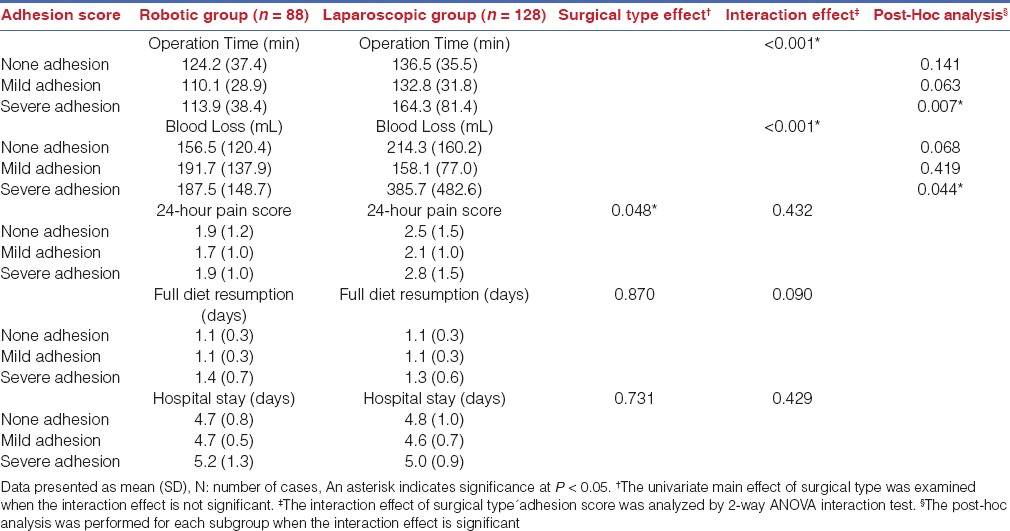
To evaluate the effects of uterine weight and surgical type to the operative outcomes, we further grouped the patients by uterine weight. Similarly, two-way ANOVA was adopted to examine the effects of covariables (surgical type and uterine weight) to each peri-operative parameters. As shown in Table 4, the interaction effect of uterine weight and surgical type was not significant for all perioperative parameters, indicating that uterine weight does not affect the surgical outcomes of both approaches. However, the main effect of surgical type for 24-h pain score was found to be significant (P=0.010). The data suggested that robotic surgery was associated with reduced postoperative pain scores, no matter the uterine weight of the cases.
Table 4.
Peri-operative parameters by the size of uterine mass
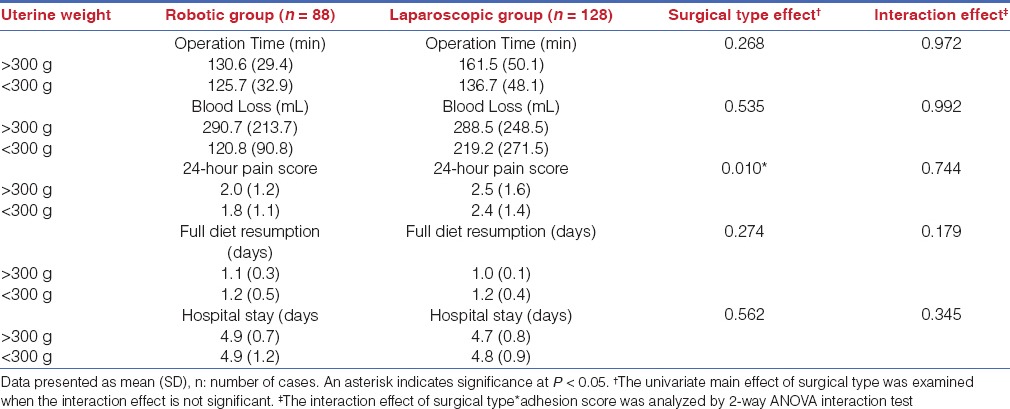
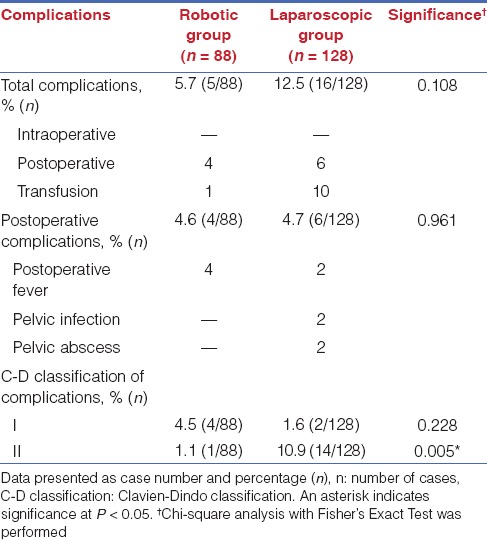
Table 5 summarized the intraoperative conversions of both surgical groups. Two patients from laparoscopic group with severe adhesions (both with uterine weight <300 g) were conversed to laparotomy (3.1%), whereas no patients in the robotic group with severe adhesions required laparotomy conversion (0%). The total and postoperative complication rate was listed in Table 6 for each group. Four cases of postoperative fever and one case of transfusion were examined in the robotic group. Two case of postoperative fever, two cases of pelvic infection, two case of pelvic abscess, and ten case of transfusion were examined in the laparoscopic group. The total complication rates were reported as 5.7% for robotic surgery versus 12.5% for laparoscopic surgery, which showed no significant difference between groups. The postoperative complication rates were 4.6% for robotic group versus 4.7% for laparoscopy group, by which significant difference was also not found between groups. However, when classified the complications by Clavien–Dindo classification, robotic group showed a lower complication rates for grade-II complications.
Table 5.
Laparotomy conversion rates between groups

Table 6.
Peri-operative complications
DISCUSSION
In this study, we compared the surgical performance of robotic surgery and laparoscopy to perform total hysterectomy. The interaction effect of pelvic adhesions and uterine weight toward each surgical parameter was examined, as long as their main effects to each perioperative factor. By our data, robotic approach showed a significantly reduced operation time and decreased blood loss only in severe adhesion group, implying that robotic surgery is a potentially superior alternative for hysterectomies complicated with severe adhesions that contraindicated to conventional laparoscopy. Moreover, robotic group showed lower pain scores than laparoscopic group independent of adhesion score or uterine weight, demonstrating that the postoperative pain is only affected by the surgical type. The conversion rates were 0% for the robotic surgery group versus 3.1% for the laparoscopy group, though the difference was not significant. Total complication rates also showed no significant difference between robotic (5.7%) and laparoscopic group (12.5%); however, when considered the grading of the complications, robotic group (1.1%) had a significant lower grade-II complication rate than the laparoscopic group (10.9%).
These results demonstrated that robotic surgery was associated with a favorable short-term surgical outcome in performing hysterectomies with severe adhesions. Interestingly, robotic approach was associated with a lower postoperative pain by our data. Hachem et al. had suggested that robotic approach showed no significant differences in postoperative pain scores and narcotic use to conventional laparoscopy for benign conditions.[30] In another aspect, Soliman et al. reported that intravenous opioids administered were significantly less for robotic surgery for cervical cancer treatment.[31] Leitao et al. also demonstrated that, for endometrial cancer management, robotic surgery was associated with a lower total fentanyl dose compared to conventional laparoscopy.[32] These outcomes imply that robotic surgery may only contribute to postoperative pain in patients receiving relative complicated surgical procedures. In this study, we used similar port number and trocar size for both approaches. A possible reason for the reduced pain is that during surgery, robotic arms pivoted at the incision sites moved and rotated around a fixed remote center to decrease the chance to use of abdominal wall for leverage, thus, reduced the tissue damage at the abdominal wall. Another reason is that robotic approach that provided a more precise dissection for pelvic adhesion cases, thus, reduced the operation time and tissue damage.
Interestingly, two patients conversed to laparotomy in the laparoscopy group were both complicated with severe adhesions but with small uterine weight (<300 g). The result implied that the factor resulted in laparotomy conversion was mainly a high adhesion score but not a large uterine weight. Combined with the data that robotic approach was associated with reduced operation time and decreased blood loss in severe adhesion group; it is suggested that robotic surgery may reduce the chance of laparotomy conversion in cases complicated with severe adhesions, though the difference in conversion rate is not significant in our study. Furthermore, robotic surgery was associated with a lower grade II complication rate, which indicated that robotic surgery could significantly decrease the need for blood transfusion also the chance of postoperative infection.
In our setting, the Endowrist instrument is a useful tool for dealing with severe pelvic adhesions. Certain strategies can be adopted to manage adhesions between uterine and bowels. For example, by bending the bipolar forceps to a 90˚ angle, surgeons could push the adherent uterus to an anterior position by the bipolar arm. An extra grasper could be applied to hold the bowels via the accessory port to help the separation of the tissues. A counter traction force then can be created between the attached tissues and to reveal the adhesive interface. By this setting, the adhesions could be dissected more efficiently without damaging the serosa of the bowel. The Endowrist instrument also facilitated dissection at difficult angles within pelvic cavity, thus, decreased the difficulties of surgery in severe adhesion conditions. These features might also contribute to a lower intraoperative conversion rate to laparotomy for severe adhesion cases.
For managing patients with large uterine weight, the camera port can be set at 6 cm above the umbilicus with the side arms set at 8 to 10 cm caudal-lateral to the scope at each side. By this setting, a larger operative field inside the abdominal cavity can be obtained, which allows a more flexible manipulation of the instruments above the uterus. This setting can also be applied for conventional laparoscopy; however, extended length laparoscopic instruments will be required.
The limitation of this retrospective study is that the cohort of patients was not unselected cases. As pelvic adhesion was suspected, surgeon might make suggestions on surgical options and the patients did self-selection to receive robotic or laparoscopic procedures. To validate the data, we carefully grouped the patients and demonstrated that robotic surgery was associated with decreased operation time and reduced blood loss in performing total hysterectomy with severe adhesions. Moreover, a lower operative pain was also observed in robotic group independent of adhesion score and uterine weight. To manage pelvic adhesions during gynecological surgery can be time consuming and technically difficult. Our data suggested that with appropriate training, robotic approach offers an superior alternative to treat severe adhesion cases, while operative time and length of hospital stay can be further decreased for experienced surgeons.[21] These results indicated that when severe adhesions were suspected in patients with prior pelvic surgeries or implied by pelvic examination, robotic surgery might be a potential alternative.
ACKNOWLEDGEMENT
This work is granted by Taipei medical university hospital (103TMU-TMUH-23).
Footnotes
Source of Support: This work is granted by Taipei medical university hospital (103TMU-TMUH-23)
Conflict of Interest: None declared.
REFERENCES
- 1.Ellis H. The causes and prevention of intestinal adhesions. Br J Surg. 1982;69:241–3. doi: 10.1002/bjs.1800690502. [DOI] [PubMed] [Google Scholar]
- 2.DiZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61:219–35. doi: 10.1016/s0015-0282(16)56507-8. [DOI] [PubMed] [Google Scholar]
- 3.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance.Recent advances in prevention and management. Dig Surg. 2001;18:260–73. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 4.Lower AM, Hawthorn RJ, Ellis H, O’Brien F, Buchan S, Crowe AM. The impact of adhesions on hospital readmissions over ten years after 8849 open gynaecological operations: An assessment from the Surgical and Clinical Adhesions Research Study. BJOG Jul. 2000;107:855–62. doi: 10.1111/j.1471-0528.2000.tb11083.x. [DOI] [PubMed] [Google Scholar]
- 5.Cho HY, Kim HB, Kang SW, Park SH. When do we need to perform laparotomy for benign uterine disease? Factors involved with conversion in vaginal hysterectomy. J Obstet Gynaecol Res. 2012;38:31–4. doi: 10.1111/j.1447-0756.2011.01657.x. [DOI] [PubMed] [Google Scholar]
- 6.Advincula AP, Song A. The role of robotic surgery in gynecology. Curr Opin Obstet Gynecol. 2007;19:331–6. doi: 10.1097/GCO.0b013e328216f90b. [DOI] [PubMed] [Google Scholar]
- 7.Sert MB, Abeler V. Robot-assisted laparoscopic radical hysterectomy: Comparison with total laparoscopic hysterectomy and abdominal radical hysterectomy; one surgeon's experience at the Norwegian Radium Hospital. Gynecol Oncol. 2011;121:600–4. doi: 10.1016/j.ygyno.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Magrina JF, Cetta RL, Chang YH, Guevara G, Magtibay PM. Analysis of secondary cytoreduction for recurrent ovarian cancer by robotics, laparoscopy and laparotomy. Gynecol Oncol. 2013;129:336–40. doi: 10.1016/j.ygyno.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012;165:289–94. doi: 10.1016/j.ejogrb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol. 2012;127:11–7. doi: 10.1016/j.ygyno.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Magrina JF, Zanagnolo V, Giles D, Noble BN, Kho RM, Magtibay PM. Robotic surgery for endometrial cancer: Comparison of perioperative outcomes and recurrence with laparoscopy, vaginal/laparoscopy and laparotomy. Eur J Gynaecol Oncol. 2011;32:476–80. [PubMed] [Google Scholar]
- 12.ElSahwi KS, Hooper C, De Leon MC, Gallo TN, Ratner E, Silasi DA, et al. Comparison between 155 cases of robotic vs. 150 cases of open surgical staging for endometrial cancer. Gynecol Oncol. 2012;124:260–4. doi: 10.1016/j.ygyno.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Fleming ND, Ramirez PT. Robotic surgery in gynecologic oncology. Curr Opin Oncol. 2012;24:547–53. doi: 10.1097/CCO.0b013e328354e572. [DOI] [PubMed] [Google Scholar]
- 14.Yim GW, Kim YT. Robotic surgery in gynecologic cancer. Curr Opin Obstet Gynecol. 2012;24:14–23. doi: 10.1097/GCO.0b013e32834daebc. [DOI] [PubMed] [Google Scholar]
- 15.Lowery WJ, Leath CA, 3rd, Robinson RD. Robotic surgery applications in the management of gynecologic malignancies. J Surg Oncol. 2012;105:481–7. doi: 10.1002/jso.22080. [DOI] [PubMed] [Google Scholar]
- 16.Tusheva OA, Gargiulo AR, Einarsson JI. Application of robotics in adnexal surgery. Rev Obstet Gynecol. 2013;6:e28–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Knox ML, El-Galley R, Busby JE. Robotic versus open radical cystectomy: Identification of patients who benefit from the robotic approach. J Endourol. 2013;27:40–4. doi: 10.1089/end.2012.0168. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Lu D, Wang L, Shi G, Song H, Clarke J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012;2:CD008978. doi: 10.1002/14651858.CD008978.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Einarsson JI. Robotic Surgery from a Laparoscopic Surgeon's Point of View. J Minim Invasive Gynecol. 2013;20:541–2. doi: 10.1016/j.jmig.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Lonnerfors C, Persson J. Implementation and applications of robotic surgery within gynecologic oncology and gynecology; analysis of the first thousand cases. Ceska Gynekol. 2013;78:12–9. [PubMed] [Google Scholar]
- 21.Woelk JL, Casiano ER, Weaver AL, Gostout BS, Trabuco EC, Gebhart JB. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121:87–95. doi: 10.1097/aog.0b013e31827a029e. [DOI] [PubMed] [Google Scholar]
- 22.Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309:689–98. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Chiu LH, Chang CW, Yen YK, Huang YH, Liu WM. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int J Gynecol Cancer. 2014;24:1105–11. doi: 10.1097/IGC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 24.Lyons TL, Adolph AJ, Winer WK. Laparoscopic supracervical hysterectomy for the large uterus. J Am Assoc Gynecol Laparosc. 2004;11:170–4. doi: 10.1016/s1074-3804(05)60193-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim HB, Song JE, Kim GH, Cho HY, Lee KY. Comparison of clinical effects between total vaginal hysterectomy and total laparoscopic hysterectomy on large uteruses over 300 grams. J Obstet Gynaecol Res. 2010;36:656–60. doi: 10.1111/j.1447-0756.2010.01185.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu WM, Wang PH, Chou CS, Tang WL, Wang IT, Tzeng CR. Efficacy of combined laparoscopic uterine artery occlusion and myomectomy via minilaparotomy in the treatment of recurrent uterine myomas. Fertil Steril. 2007;87:356–61. doi: 10.1016/j.fertnstert.2006.07.1497. [DOI] [PubMed] [Google Scholar]
- 27.Uccella S, Ceccaroni M, Cromi A, Malzoni M, Berretta R, De Iaco P, et al. Vaginal cuff dehiscence in a series of 12,398 hysterectomies: Effect of different types of colpotomy and vaginal closure. Obstet Gynecol. 2012;120:516–23. doi: 10.1097/AOG.0b013e318264f848. [DOI] [PubMed] [Google Scholar]
- 28.Improvement of interobserver reproducibility of adhesion scoring systems. Adhesion Scoring Group. Fertil Steril. 1994;62:984–8. [PubMed] [Google Scholar]
- 29.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Hachem L, Acholonu UC, Jr, Nezhat FR. Postoperative pain and recovery after conventional laparoscopy compared with robotically assisted laparoscopy. Obstet Gynecol. 2013;121:547–53. doi: 10.1097/AOG.0b013e318280da64. [DOI] [PubMed] [Google Scholar]
- 31.Soliman PT, Langley G, Munsell MF, Vaniya HA, Frumovitz M, Ramirez PT. Analgesic and antiemetic requirements after minimally invasive surgery for early cervical cancer: A comparison between laparoscopy and robotic surgery. Ann Surg Oncol. 2013;20:1355–9. doi: 10.1245/s10434-012-2681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitao MM, Jr, Malhotra V, Briscoe G, Suidan R, Dholakiya P, Santos K, et al. Postoperative pain medication requirements in patients undergoing computer-assisted (“Robotic”) and standard laparoscopic procedures for newly diagnosed endometrial cancer. Ann Surg Oncol. 2013;20:3561–7. doi: 10.1245/s10434-013-3064-9. [DOI] [PubMed] [Google Scholar]


