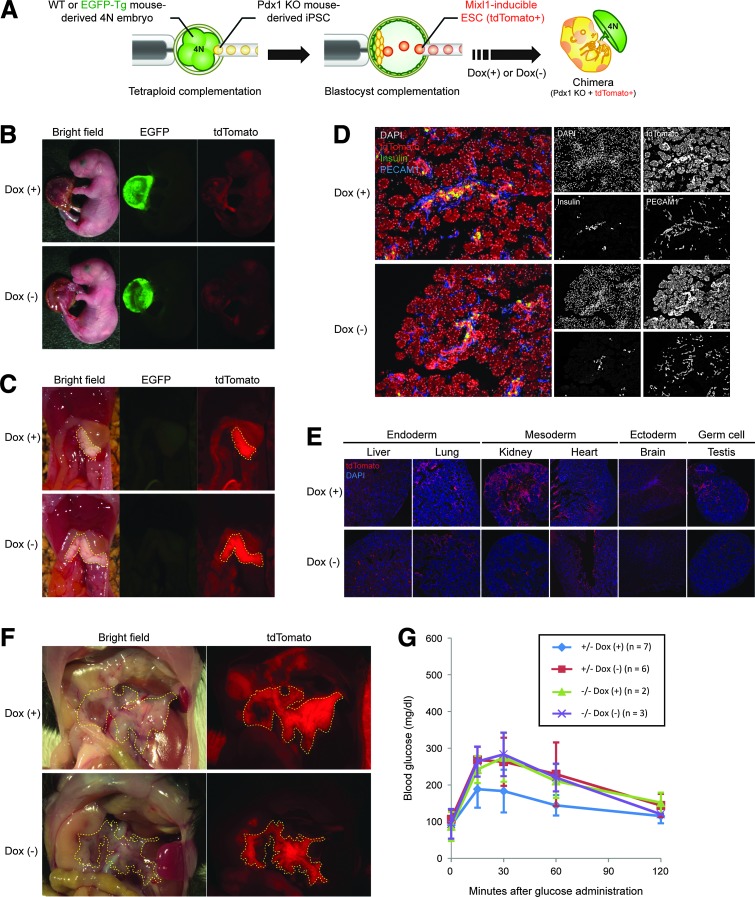
FIG. 3.
Blastocyst complementation using Mixl1-inducible cells enables generation of functional pancreata in vivo. (A) Schema of novel blastocyst complementation system using tetraploid complementation and Mixl1-inducible ESCs. (B) Neonates generated via method shown in (A). To make tetraploid embryos, two-cell stage embryos derived from transgenic mice that ubiquitously express EGFP (EGFP-Tg) were electrofused. In the Dox(−) setting, foster mothers were given drinking water without Dox until 4 days after embryo transfer and were then given water with Dox. (C) Macroscopic views of internal organs of neonates shown in (B). Yellow dashes outline pancreata entirely composed of CHT5-ESC–derived cells. (D) Microscopic views of distribution of CHT5-ESC–derived cells in pancreata of newborn pups. Sections were immunostained for DsRed (red), insulin (green), and PECAM-1 (blue), with nuclear counterstaining using DAPI (white). (E) Microscopic views of distribution of CHT5-ESC–derived cells in various tissues of newborn pups. Sections were immunostained for DsRed (red), with nuclear counterstaining using DAPI (blue). (F) Macroscopic views of adult pancreata generated by an injection of CHT5-ESCs into Pdx1−/− blastocysts. In both Dox(+) and Dox(−) settings, mice have pancreata completely derived from CHT5-ESCs expressing tdTomato. (G) Results of glucose tolerance testing in Pdx1−/− and Pdx1+/− mice complemented with CHT5-ESCs in Dox(+) or Dox(−) settings (see insert). Blood was sampled via tail vein at intraperitoneal glucose administration (1 g/kg; 0 min) and at 15, 30, 60, and 120 min thereafter. Sections in (D, E) were observed under confocal laser scanning microscopy.