Fig. 2. Temperature-dependent transactivation by Krox20 and E2F4 in tsBN67 cells.
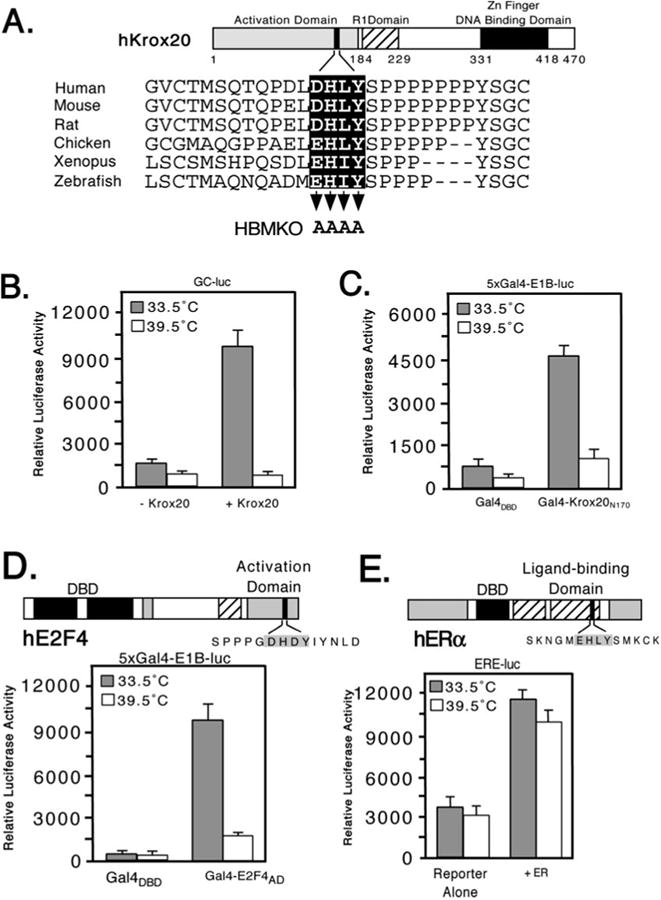
A, schematic showing structure of murine Krox20 with sequences of the HBM region from the following orthologs: Zebrafish (SWISS-PROT accession Q05159), Xenopus (Q08427), chicken (Q98T82), rat (P51774), mouse (P08152), and human (P11161). Three functional domains have been defined: an N-terminal activation domain (residues 1–184), a repression domain (193–229), and a DNA-binding domain composed of three zinc fingers (331–418). Residues mutated to alanine in Krox20 HBMKO are indicated by arrowheads. B, hamster tsBN67 cells were cotransfected with an expression plasmid (500 ng) encoding full-length mouse Krox20 together with a Krox20 responsive reporter (GC-luc, 5 μg) and incubated at 33.5 or 39.5 °C for 40 h. Each assay was performed in triplicate and the mean ± S.D. are shown. C, as in panel B, except that tsBN67 cells were cotransfected with 500 ng of expression plasmid encoding Gal4DBD or Gal4-Krox20N170 together with a Gal4 reporter (5xGal4-E1B-luc, 500 ng). D, domain structure of human E2F4. The HBM (filled box) lies within the transactivation domain (33). For the reporter assay tsBN67 cells were cotransfected with 250 ng of Gal4DBD or Gal4-E2F4-(240–412) expression plasmid and 500 ng of Gal4-luciferase reporter. E, in the estrogen receptor (ERα) the putative HBM (filled box) lies in the ligand-binding domain. 500 ng of ERα expression plasmid were cotransfected into tsBN67 cells together with an ER-responsive reporter plasmid (ERE-luc, 500 ng). Note that the calf serum used to culture the cells contains sufficient levels of natural ligands to activate the transfected receptors.
