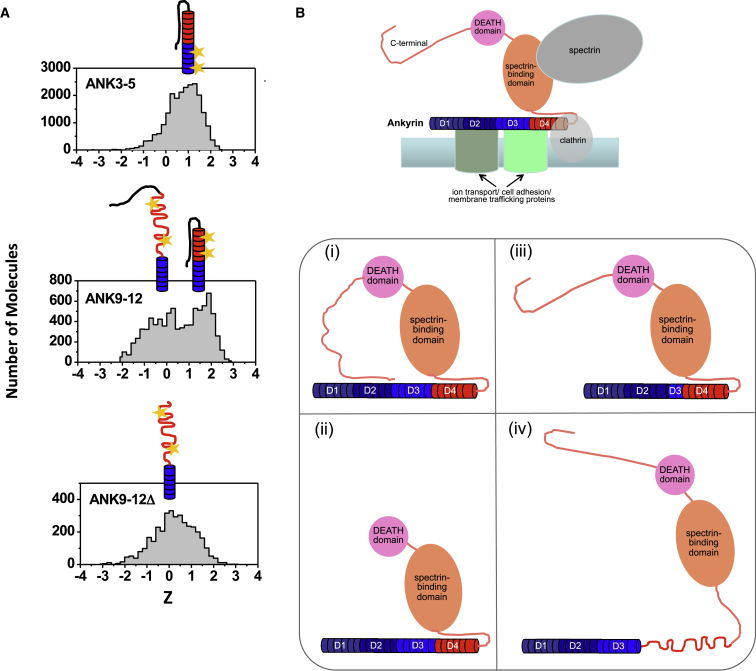
Figure 3.
Schematics Summarizing the Main Findings of the Single-Molecule Analysis and Their Functional Implications
(A) Schematic of the energy landscape of D34 resolved by smFRET. The unstructured loop can adopt two different arrangements under native conditions, giving rise to distinct FRET populations.
(B) Schematic showing the mechanisms by which Ankyrins are auto-regulated. (Top) Ankyrin domain structure and binding of partner proteins. The MBD comprises 24 ankyrin repeats in four subdomains, D1–D4, the C-terminal 12 of which constitute D34. For simplicity, the ankyrin repeats are drawn with a linear shape, although they in fact form a superhelical spiral. The MBD and SBD are intimately associated via an unstructured loop from the latter domain that packs back against the six most C-terminal ankyrin repeats of the former domain. (Bottom) Multiple mechanism operate to regulate and diversify Ankyrin functions: (i) intramolecular association of the C-terminal regulatory domain and the MBD; alternative splicing, which, for example, leads to deletion of (ii) the C-terminal regulatory domain or (iii) individual ankyrin repeats; and (iv) folding/unfolding of the C-terminal ankyrin repeats, controlled by the unstructured segment from the SBD acting as a molecular safety pin or staple, which will further modulate the anchoring activity of Ankyrins.