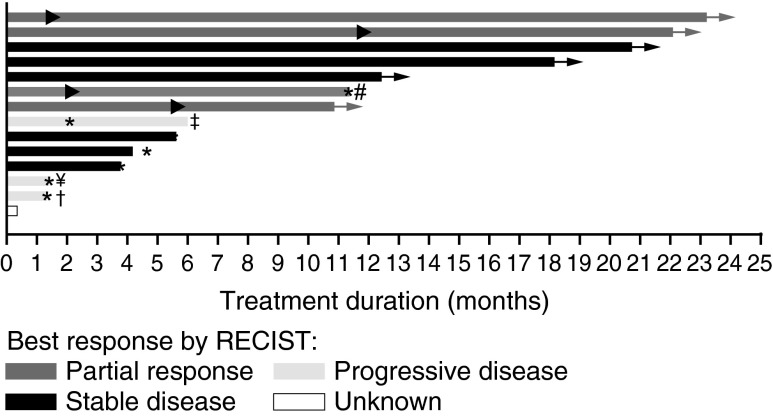
FIG. 1.
Duration on treatment for 14 patients with BRAFV600E-mutant thyroid cancer treated with dabrafenib. ▸, indicates the first occurrence of PR; *, indicates time of PD;→, patient continued treatment in the rollover study; ¥, patient received prior MEK inhibitor treatment; #, patient received 100 mg TID, all other subjects received 150 mg BID; †, patient with concurrent papillary and Hurthle cell features; ‡, patient with anaplastic histology. This patient had a 66% decrease in target lesions but developed a new lesion at first restaging. Note 1: Six patients had not developed progressive disease at the time of study completion and continued receiving treatment in a rollover study. At the time of this publication, all six of these patients are still receiving dabrafenib, and four of them have been receiving dabrafenib for more than 2.5 years. Note 2: One patient withdrew consent before having any post-baseline disease assessment. Note 3: Duration on treatment is defined as the time interval from the first dose to the last dose on the phase 1 study, regardless of dose interruption. BID, twice a day; PD, progressive disease; PR, partial response; SD stable disease; TID, three times a day; UNK, unknown response.