Figure 4. hIgG strongly inhibits NMO-IgG-mediated CDC and the classical complement pathway.
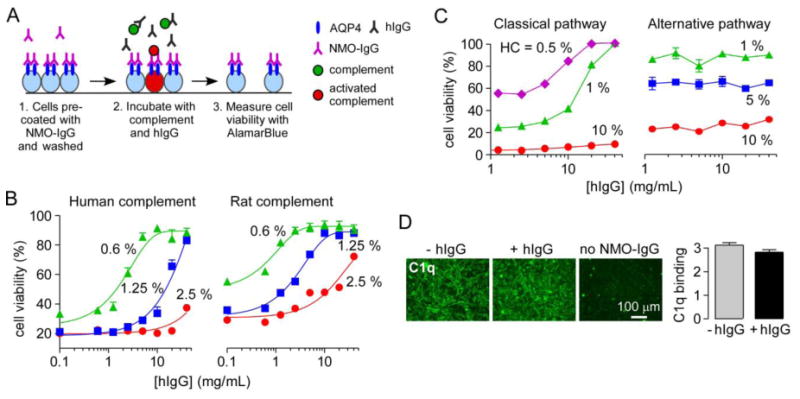
A. Experimental protocol to measure complement-dependent cytotoxicity in NMO-IgG-coated CHO-AQP4 cells. CHO-AQP4 cells were incubated with 20 μg/mL rAb-53 for 1 h at 23 °C and washed extensively. Complement was pre-incubated for 1 h at 4 °C with different concentrations of hIgG and then added to the NMO-IgG-coated CHO-AQP4 cells for 1 h at 23 °C. Target cells were then washed extensively in PBS and viability was measured by addition of 20% AlamarBlue for 1 h at 37 °C. B. Cell viability as a function of hIgG concentration for indicated concentrations of human or rat complement (mean ± S.E., n=3). C. Cell viability in IgM-coated sheep red blood cells (assay of classical pathway) and uncoated rabbit red blood cells (assay of alternative pathway) for different concentrations of human complement (HC) (mean ± S.E., n=3). D. C1q binding to NMO-IgG-coated CHO-AQP4 cells with or without 40 mg/mL hIgG. Graph shows C1q binding as fluorescence intensity (mean ± S.E., n=5).
