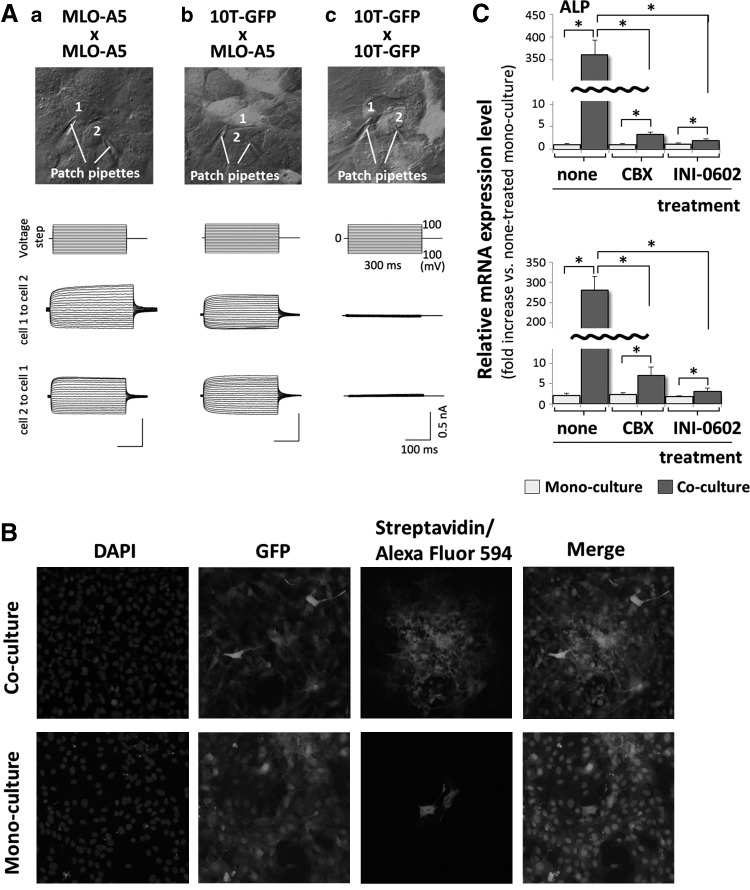
FIG. 6.
ALP and BSP mRNA expression in 10T-GFP cells is triggered via gap junction-mediated intercellular communication with bone marrow mesenchymal stem cells (BMSCs). (A) Patch clamp assays showing the passage of electrical current between pairs of MLO-A5 and MLO-A5 cells (a), 10T-GFP and MLO-A5 cells (b), and 10T-GFP and 10T-GFP cells (c) that were cultured for 24 h before analysis. The cells with the attached patch pipettes are numbered. (B) Fluorescence microscopy images of biocytin-injected 10T-GFP and MLO-A5 cells. 10T-GFP cells were mono-cultured or co-cultured with MLO-A5 cells at 24 h and then, biocytin was injected into a pair of adjacent 10T-GFP and MLO-A5 cells, or a pair of adjacent mono-cultured 10T-GFP cells. The cells were cultured for another 6 h, fixed, and then stained with DAPI to detect the nuclei. EGFP expressing cells were also detected. Biocytin transfer between neighboring cells was detected using Alexa Fluor 594-conjugated streptavidin. (C) Real-time RT-PCR analyses of on the expression levels of the ALP and BSP mRNAs in 10T-GFP cells that were incubated in the presence or absence of carbenoxolone (CBX) (100 μM) or INI-0602 (100 μM) for 1 h and mono-cultured or co-cultured with MLO-A5 cells for 12 h. The expression level of each mRNA was normalized to that in the nontreated mono-cultured cells, and the data are represented as the mean±SD of n=3 replicates. *P<0.05.