Figure 5. Poly-ubiquitination in the cytoplasmic vesicle regulates PAPC localization.
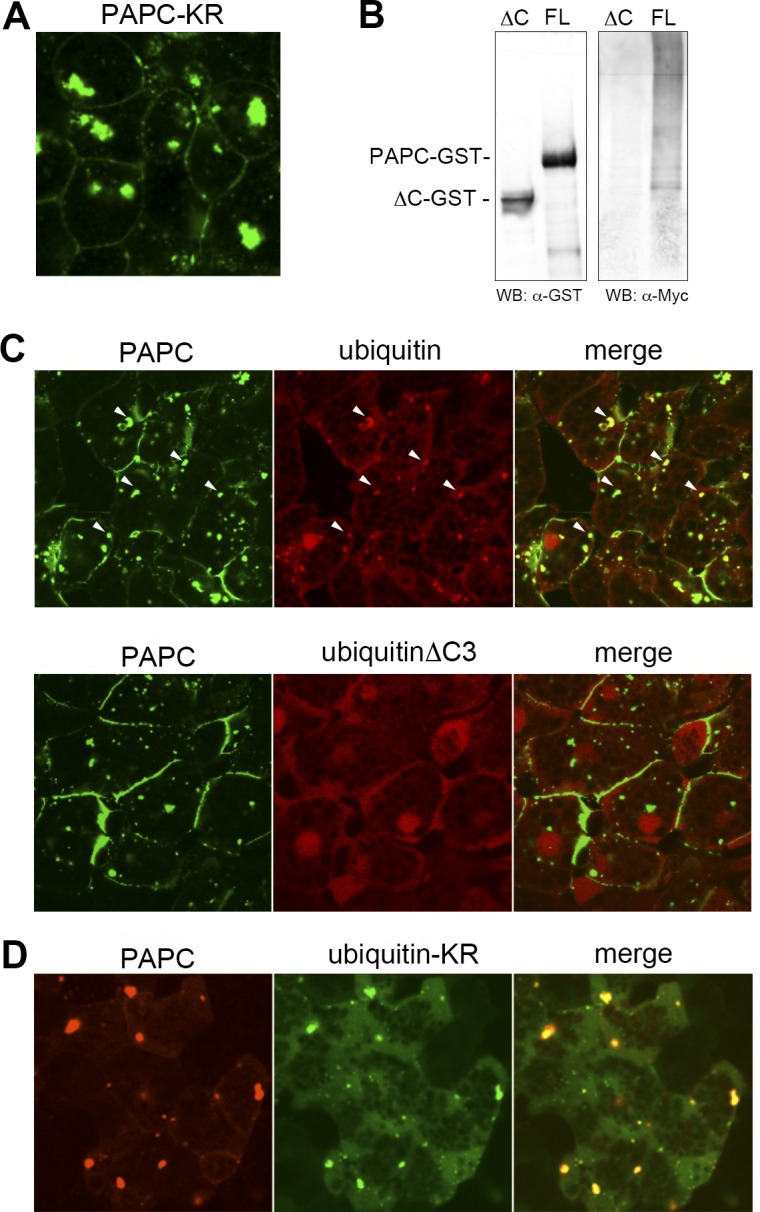
(A) Changing nine lysine residues in the cytoplasmic region of PAPC to arginine resulted in PAPC aggregation in the cytoplasm, indicating the role of ubiquitination in PAPC localization. (B) The ubiquitination assay revealed that PAPC is poly-ubiquitinated in the Xenopus embryo. Myc-tagged ubiquitin co-expressed with GST-tagged full-length PAPC (FL) in the Xenopus embryo was purified with PAPC-GST and detected as a high molecular weight ladder. Deletion of PAPC cytoplasmic domain (ΔC) diminished poly-ubiquitination. (C) RFP-tagged ubiquitin and GFP-tagged PAPC co-localized in the cytoplasmic vesicle in animal cap cells (arrowheads). By contrast, RFP-tagged UbiquitinΔC3, which has three amino acids at the C-terminus removed and therefore cannot be conjugated to the target, did not co-localize with PAPC-GFP in animal cap cells. GFP-tagged ubiquitin-KR, which is conjugated to the target but blocks formation of poly-ubiquitin, co-localized with PAPC-RFP in the cytoplasmic vesicles. Ubiquitin-KR-GFP-expressing cells from which PAPC had been depleted from the plasma membrane exhibited a round morphology indicative of reduced cell-cell adhesion.
