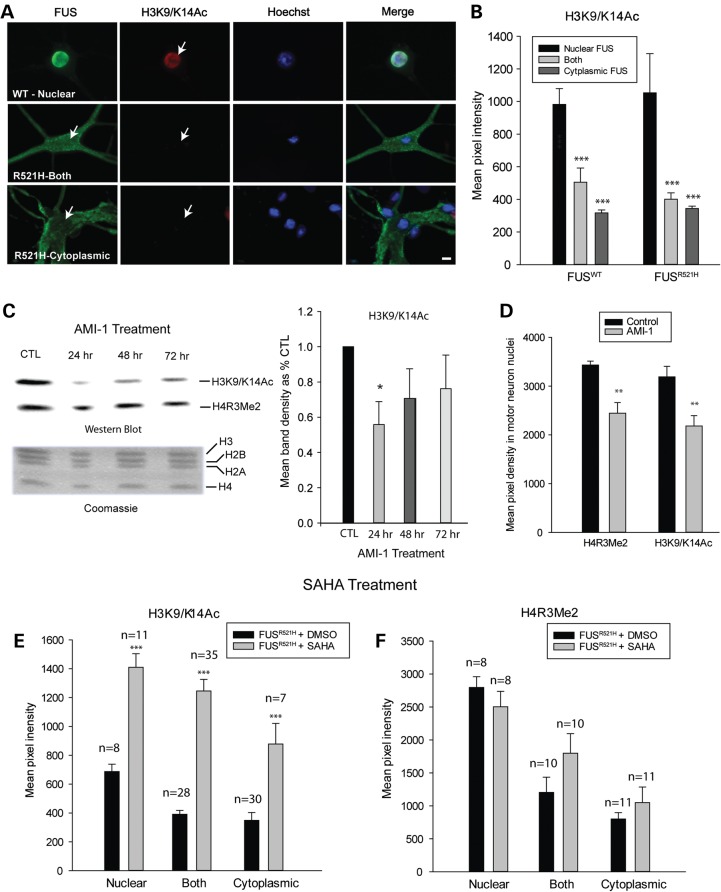
Figure 4.
H3K9/K14 acetylation is decreased in neurons with cytoplasmic FUS, downstream of H4R3 methylation. (A) Double labeling of motor neurons, expressing either WT or mutant FUS, with antibodies to FLAG and acetylated H3K9/K14 (H3K9/K14Ac) demonstrating decreased H3K9/K14 acetylation in neurons with cytoplasmic FUS. Arrows point to motor neuron nuclei. Presented are means ± SE of data collected in n = 43 neurons expressing WT FUS and n = 32 neurons expressing R521H FUS. (B) Mean fluorescence intensity of H3K9/K14Ac labeling corresponding to localization of FUS. Asterisks indicate significantly different compared with neurons with nuclear FUS, ***P < 0.001. (C) Western blot of purified histones from cultures treated with 20 µm AMI-1 (PRMT inhibitor), demonstrating a significant decrease in H3 acetylation, indicated by densitometric measurements of H3K9/K14Ac bands (n = 3 per condition), at 24 h of treatment. Coomassie-stained sister gel shows consistent levels of histone proteins among treatment groups. Asterisks indicate significantly different compared with untreated cultures, *P < 0.05. (D) Reduction in H4R3Me2 and H3K9/K14Ac in motor neurons after 24 h of exposure to 20 µm AMI-1 (**P = 0.01), presented as mean fluorescence intensity ± SE of antibody labeling in nuclei of motor neurons with nuclear FUS. (E and F) Mean fluorescence intensity of H3K9/K14Ac and H4R3Me2 labeling of neurons expressing R521H FUS in control cultures (vehicle treated) or cultures treated with the HDAC inhibitor, SAHA. SAHA significantly preserved H3 acetylation but did not prevent decreases in H4R3 methylation in neurons with cytoplasmic FUS. Asterisks indicate significantly different compared with vehicle treated ***P < 0.001; number of neurons evaluated is indicated on the graphs.