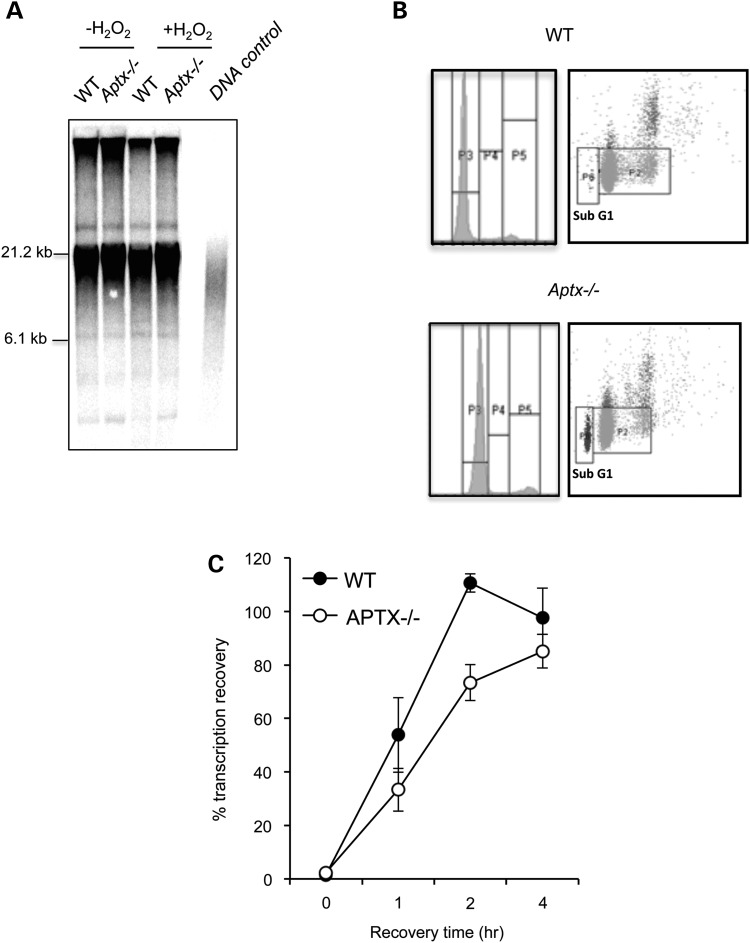
Figure 2.
Aptx−/− cells show no detectable defect in telomere length but reduced transcription recovery following oxidative stress. (A) WT and Aptx−/− MEFs (passage 8) were mock incubated or incubated with pulsed treatment of 50 μm H2O2 for 10 min over three consecutive days. Genomic DNA was extracted and non-telemetric DNA was enzymatically digested and resolved by gel electrophoresis. Southern blotting was performed using a digoxigenin (DIG)-labelled probe specific for telomeric repeats (Roche) and incubated with a DIG antibody coupled to alkaline phosphatase. Telomeric signal was detected by chemiluminescence. Telomeric DNA control was obtained from Roche and used as size markers (not shown). (B) MEFs were fixed with cold ethanol, stained with propidium iodide and subjected to FACS analysis. A representative FACS profile is depicted. (C) The indicated MEFs were plated onto 3.5 cm dishes at 2 × 104 cells per ml in medium containing 0.02 µCi/ml thymidine [2-14C] and incubated at 37°C for 48 h (∼2 cycles) to label DNA. Thymidine [2-14C] was removed and cells chased for 4 h and then mock treated or treated with 50 µM H2O2 for 10 min at room temperature. RNA synthesis was measured by incubation with 5 µCi/ml 3H-uridine for 15 min immediately after treatment or following subsequent incubation in H2O2-free media for the indicated recovery periods. RNA was quantified using Beckman Coulter LS6500 Scintillation Counter. RNA synthesis (3H count) was normalized to DNA content (14C count) and data plotted as % RNA synthesis relative to control un-treated samples from three biological replicates ± SEM.