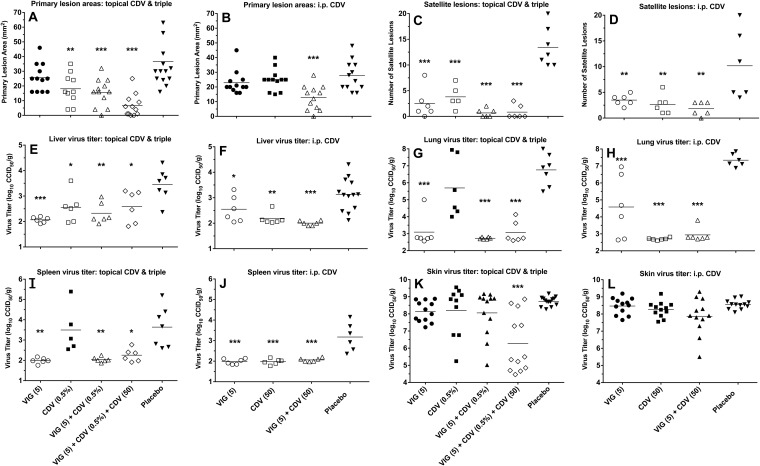
FIG 4.
Effects of topical CDV, parenteral CDV, and VIG (each used alone and in double or triple combination) treatments on primary lesion areas (A and B), numbers of satellite lesions (C and D), and tissue virus titers (E through L) on day 12 of a cutaneous vaccinia virus infection in cyclophosphamide-immunosuppressed SHK-1 hairless mice. The treatment regimens were the same as those described for Fig. 1. Mean values are represented by the horizontal bars. Six mice were in each treatment group. With two primary lesions per mouse, there were 12 measurements for the primary lesion areas and the skin virus titers. Open symbols represent data sets that were significantly different than those in the placebo group. *, P < 0.05, **, P < 0.01, and ***, P < 0.001. CCID50, 50% cell culture infectious dose.