FIG 5.
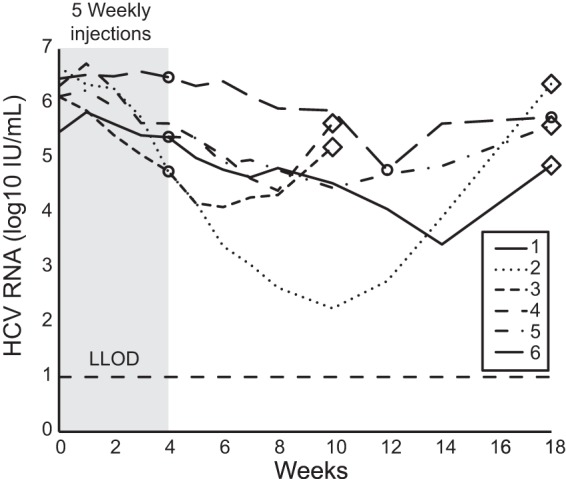
Subjects with chronic HCV genotype 1 infection were randomly assigned to receive five weekly subcutaneous injections of miravirsen at doses of 3 mg, 5 mg, or 7 mg per kilogram of body weight over a 29-day period (gray shading) (33). They were monitored for 18 weeks after randomization. Amplification and sequence analysis of the entire 5′UTR (nucleotides 1 to 341) were performed by 5′ RACE from six subjects (numbered 1 to 6) who experienced virologic rebound. Identification of the C3U nucleotide change (large open triangles) or wild-type sequences at position 3 (large open circles) are indicated. The lower limit of detection (LLOD) was 12 IU (1.08 log10 IU per ml).
