Abstract
Molecular chaperones reside in nearly every organelle within a eukaryotic cell, and in each of these compartments, they ensure that protein homeostasis (or proteostasis) is maintained. In this issue, Wiseman and colleagues find that an ER lumenal chaperone escapes this compartment when a specific stress pathway is activated. The chaperone, an Hsp40 homolog known as ERdj3, transits through the secretory pathway to the extracellular space. During this journey, ERdj3 can escort an aggregation-prone protein or it can identify aggregation-prone proteins extracellularly, thereby functioning outside of its normal environment.
See also: JC Genereux et al (January 2015)
Molecular chaperones augment the folding of newly synthesized proteins, the refolding of damaged proteins, the trafficking of proteins to intracellular organelles, and the delivery of misfolded proteins to degradative systems (Kim et al, 2013). When overwhelmed, specific cellular pathways are triggered that aid the chaperone network. For example, in the endoplasmic reticulum (ER), an increase in the concentration of misfolded proteins triggers the unfolded protein response (UPR), which in turn increases the synthesis of factors that enlarge the ER and those that post-translationally modify and deliver misfolded proteins for ER-associated degradation (ERAD) or autophagy (Walter & Ron, 2011). The production of specific molecular chaperones in the ER is also induced by the UPR since they prevent protein aggregation and facilitate protein folding. It has been commonly believed that these chaperones act solely within ER, performing a dedicated task in the protein folding assembly line and then lying in wait for the next substrate. In this issue of The EMBO Journal, Wiseman and colleagues (Genereux et al, 2015) report that induction of one leg of the UPR leads to the secretion of an ER resident chaperone, one that has the ability to escort an aggregation-prone protein through the secretory pathway and out of the cell (where it may be less toxic). After secretion, the chaperone can also associate with aggregation-prone proteins, thus acting as a regulator of extracellular proteostasis.
One target of the UPR is an ER lumenal Hsp40, ERdj3, which functions as a cochaperone for BiP, an ER lumenal Hsp70. ERdj3 binds to misfolded proteins and can escort misfolded proteins to BiP and stimulate BiP's ATPase activity (Shen & Hendershot, 2005). These events facilitate protein folding. ERdj3 is also required to target select, misfolded proteins for ERAD (Buck et al, 2010). However, unlike most ER resident chaperones, ERdj3 lacks a C-terminal ER retention motif (KDEL).
Based on these data, Genereux et al hypothesized that ERdj3 might function extracellularly. Therefore, they assayed media collected from human cell lines after ER stress was induced with a chemical modulator. Up to 50% of newly synthesized ERdj3 was found in the extracellular media. Two other stress-inducible, ER lumenal chaperones (BiP and HYOU1/GRP170) were absent from media, indicating that not all UPR-responsive chaperones are secreted in response to stress. The authors also found that the trafficking inhibitor, brefeldin A, blocked ERdj3 secretion, suggesting that ERdj3 trafficking proceeds through the canonical secretory pathway. Consistent with the idea that the UPR augments ERdj3 secretion, serum levels of ERdj3 increased in mice on a diet that induces ER stress.
The next goal was to define which leg of the UPR facilitated ERdj3 trafficking. Two transcription factors that control the UPR in mammalian cells are XBP1 and ATF6. Using an engineered cell expression system where XBP1 and/or ATF6 can be selectively activated in the absence of ER stress (Shoulders et al, 2013), the authors determined that activation of either factor increased ERdj3 levels. However, ERdj3 secretion was observed exclusively in response to ATF6 activation.
Earlier work demonstrated that ER stress results in the secretion of ER resident chaperones in yeast (Belden & Barlowe, 2001) and mammalian cells (Jordan & Gibbins, 2006), but whether this arose from the ER simply being overwhelmed by unfolded proteins or whether chaperone section might be purposeful was unexamined. Therefore, Genereux et al examined the effects of a toxic prion protein (TPrP) on PK1 mouse neuroblastoma cells treated with media from cells overexpressing ERdj3. As hoped, ERdj3-containing media reduced TPrP toxicity, whereas treatment with media from cells depleted of ERdj3 actually increased TPrP toxicity. The authors then asked whether ERdj3 acts exclusively in an extracellular manner or does it traffic in association with misfolded proteins that arise in the ER. To address this question, the association of ERdj3 with a known intracellular ERdj3 substrate, an amyloidogenic secreted protein transthyretin A25T (TTRA25T), was assayed (Shoulders et al, 2013). ERdj3 binding to TTRA25T was examined when ERdj3 and TTRA25T were co-expressed or after the media from cells expressing either ERdj3 or TTRA25T were mixed. Co-immunoprecipitation between ERdj3 and TTRA25T was observed only when ERdj3 and TTRA25T were expressed in the same cell, strongly suggesting that ERdj3 traffics with TTRA25T through the secretory pathway (Fig 1). In contrast, ERdj3 association with a stable version of TTR (M-TTR) was lower than with TTRA25T, and the known TTR stabilizer, Tafamidis (Bulawa et al, 2012), reduced the interaction between ERdj3 and TTRA25T. Finally, ERdj3 failed to promote the trafficking of a highly unstable TTR variant, TTRD18G, which interacts with ERdj3 in the ER but is instead targeted for ERAD. Together, these data support a role for ERdj3 in both stabilizing and trafficking TTRA25T through the secretory pathway.
Figure 1. ERdj3 traffics with chaperones and a misfolded form of transthyretin (TTR).
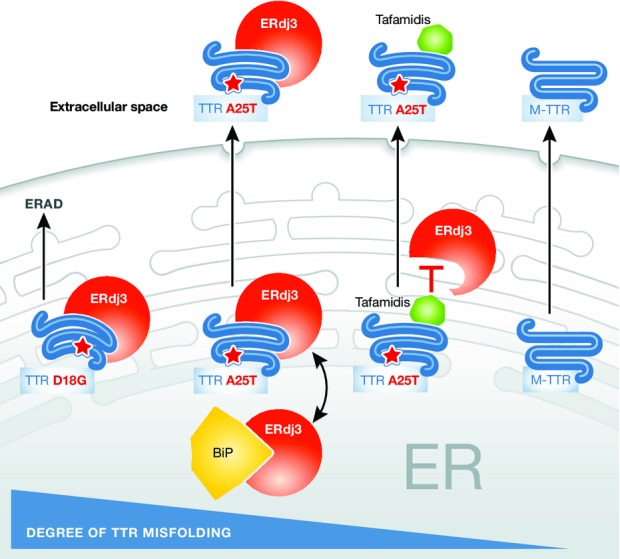
TTRD18G is a highly unstable variant targeted for ERAD by ERdj3. In contrast, another unstable variant, TTRA25T, is stabilized in the ER and escorted through the secretory pathway by ERdj3 unless the small molecule corrector, Tafamidis, is present. M-TTR is the stable, wild-type TTR variant that traffics without the assistance of ERdj3. Please note that wild-type TTR forms a tetramer, but the oligomeric state of ERdj3-stabilized TTR is unknown.
Finally, Genereux et al investigated the relationship between ERdj3 and BiP during TTRA25T quality control and secretion. The authors found that BiP overexpression reduced the amount of the secreted ERdj3-TTRA25T complex, which is consistent with BiP titrating ERdj3 from TTRA25T. This result also suggested that the role of ERdj3 in extracellular proteostasis is BiP independent. Consistent with this hypothesis, secretion of an ERdj3 mutant that is unable to interact with or stimulate the ATPase activity of BiP was unaffected by increased BiP expression.
The results presented in this paper open up several new research directions. For example, do other ER chaperones function similarly? Also, why do both XBP1 and ATF6 increase the levels of ERdj3, but only ATF6 augments chaperone secretion? Further, what is the relationship between ERdj3 and clusterin? The authors report that ERdj3 and clusterin, both of which are ATP-independent chaperones, exhibit unique effects on TTR secretion. Clusterin associates extracellularly with the β(1–40) amyloid peptide, altering its oligomeric properties (Narayan et al, 2011), and unlike ERdj3, its extracellular levels are down-regulated by the UPR (Nizard et al, 2007). Do ERdj3 and clusterin work together under any circumstances, but do they only chaperone unique substrates? More generally, these data provide additional support for the notion that the UPR might be chemically modulated to combat a range of human diseases, an effort that is underway in many laboratories.
Acknowledgments
Studies on protein quality control in the laboratory were supported by NIH grants DK090195 (TEB), GM075061 and DK079307 (JLB) and by a grant from Cystic Fibrosis Foundation Therapeutics.
References
- Belden WJ, Barlowe C. Deletion of yeast p24 genes activates the unfolded protein response. Mol Biol Cell. 2001;12:957–969. doi: 10.1091/mbc.12.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck TM, Kolb AR, Boyd C, Kleyman TR, Brodsky JL. The ER associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol Biol Cell. 2010;21:1047–1058. doi: 10.1091/mbc.E09-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, Packman J, Powers ET, Wiseman RL, Foss TR, Wilson IA, Kelly JW, Labaudiniere R. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109:9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genereux JC, Qu S, Zhou M, Ryno LM, Wang S, Shoulders MD, Kaufman RJ, Lasmézas CI, Kelly JW, Wiseman RL. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015;34:4–19. doi: 10.15252/embj.201488896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal. 2006;8:312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, Meehan S, Wilson MR, Dobson CM, Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1-40) peptide. Nat Struct Mol Biol. 2011;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizard P, Tetley S, Le Drean Y, Watrin T, Le Goff P, Wilson MR, Michel D. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8:554–565. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Hendershot LM. ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP's interactions with unfolded substrates. Mol Biol Cell. 2005;16:40–50. doi: 10.1091/mbc.E04-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR, III, Su AI, Kelly JW, Wiseman RL. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013;3:1279–1292. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]


