Figure 2. ERdj3 is efficiently secreted from cells following stress-independent activation of the UPR-associated transcription factor ATF6.
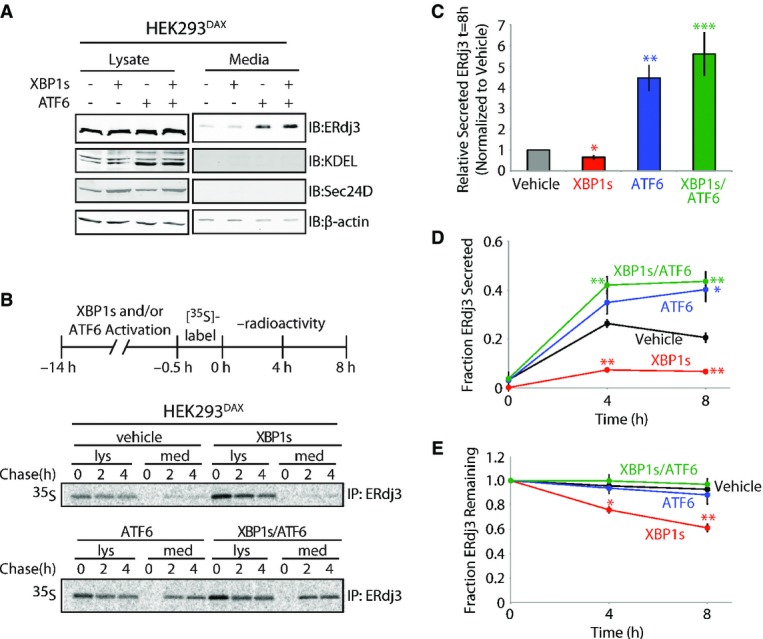
- A Immunoblot of lysates and media collected from HEK293DAX cells following 16 h of activation of XBP1s (by 1 μg/ml dox) and/or ATF6 (by 10 μM TMP), as indicated (Shoulders et al, 2013). Immunoblots of the ATF6 target protein BiP (reactive with the KDEL antibody) and the XBP1s target Sec24D confirm the small-molecule activation of these transcription factors.
- B Representative autoradiogram of [35S]-labeled ERdj3 immunopurified from lysates and media collected from HEK293DAX cells following preactivation of XBP1s (by 1 μg/ml dox) and/or ATF6 (by 10 μM TMP) (Shoulders et al, 2013). The experimental protocol is shown above.
- C Quantification of relative amounts of [35S]-labeled ERdj3 in media at 8 h collected from cells treated as shown in (B) (n = 3). The media [35S]-labeled ERdj3 was measured by densitometry and is normalized to the amount of media [35S]-labeled ERdj3 at 8 h from vehicle-treated cells.
- D, E Quantification of the fraction ERdj3 secreted (D) and the fraction ERdj3 remaining (E) from autoradiograms as shown in (B). The fractions of ERdj3 secreted/ERdj3 remaining were calculated by normalizing the recovered [35S]-labeled ERdj3 signal in the media and in the lysates at t = 4 or 8 h to the total amount of [35S]-labeled ERdj3 signal in the media and lysates at t = 0 h.
Data information: Error bars represent the standard error from biological replicates (n = 3). *P-value < 0.05; **P-value < 0.01; ***P-value < 0.001 as compared to the vehicle-treated condition.
Source data are available online for this figure.
