Figure 3. A 20-aa peptide binds to the extracellular domain of PRK5.
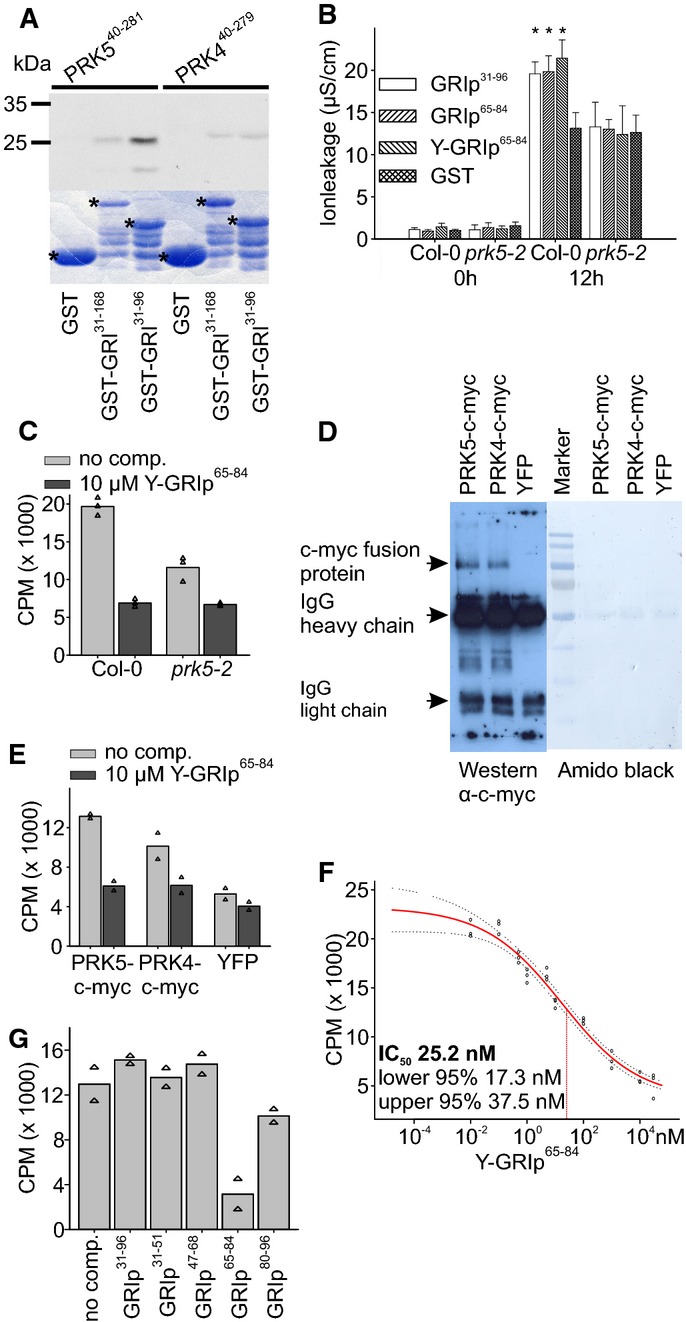
- In vitro-produced 35S-labeled ectodomains of PRK5 (PRK540–281) or PRK4 (PRK440–279) were incubated with bacterially produced GST, GST-GRI or GST-GRI31–96 and purified. GST-GRI31–96 but not GST directly bound to the ectodomain of PRK5. Binding of GST-GRI to PRK5 ectodomain and binding of GST-GRI31–96 and GST-GRI to the ectodomain of PRK4 were strongly reduced. Upper part: autoradiograph, lower part: Coomassie-stained 12% SDS–polyacrylamide gel. Asterisks in the Coomassie-stained gel indicate GST, GST-GRI and GST-GRI31–96, respectively. Supplementary Fig S12 shows a Western blot of the GST-tagged proteins.
- Infiltration of 37 nM GST, GRIp31–96, GRIp65–84 or Y-GRIp65–84 into Col-0 or prk5-2 leaves. Tyrosine-labeled GRIp65–84 still induced cell death in Col-0 but not in prk5-2 plants.
- 125I-labeled Y-GRIp65–84 (0.46 nM) bound specifically to Col-0 membrane fractions (light gray bars), the binding was significantly reduced in prk5 plants. Excess of non-radioactive Y-GRIp65–84 (10 μM) reduced binding to background levels (dark gray bars; all bars show the average of three samples, triangles show individual data points).
- Immunoprecipitation of PRK5-c-myc, PRK4-c-myc or YFP expressed in protoplasts with rabbit polyclonal anti-c-myc antibody, blotted with mouse monoclonal anti-c-myc antibody.
- Binding of 125I-Y-GRIp65–84 (0.46 nM) to immunoprecipitates (using anti-c-myc antibodies) from prk5-2 protoplasts transfected with PRK5-c-myc, PRK4-c-myc or YFP, respectively. Binding was competed out with 10 μM unlabeled Y-GRIp65–84. Bars show the average of two samples, and triangles show individual data points. Western blot is shown in panel (D).
- Analysis of 125I-Y-GRIp65–84 (0.46 nM) binding competed out with increasing amounts of unlabeled Y-GRIp65–84 to Col-0 membrane extracts. Fifty percent inhibition (IC50) occurred at 25.2 nM. Red line shows binding average competition according to a sigmoid curve, dotted lines show 95% confidence intervals, and circles show data points.
- Excess of GRIp65–84 (10 μM) but not of GRIp31–96 or other peptides (GRIp31–51, GRIp47–68, GRIp80–96) competed the binding of 0.46 nM 125I-Y-GRIp65–84 to membrane extracts from Col-0 (all bars show the average of two samples, triangles show individual data points).
Data information: Data in (B) are shown as average ± SD of four replicates consisting of four leaf disks each. Asterisks in (B) mark statistically significant differences from infiltration with GST according to Sidak’s test (P < 0.05). All experiments were repeated three times with similar results. Source data are available online for this figure.
