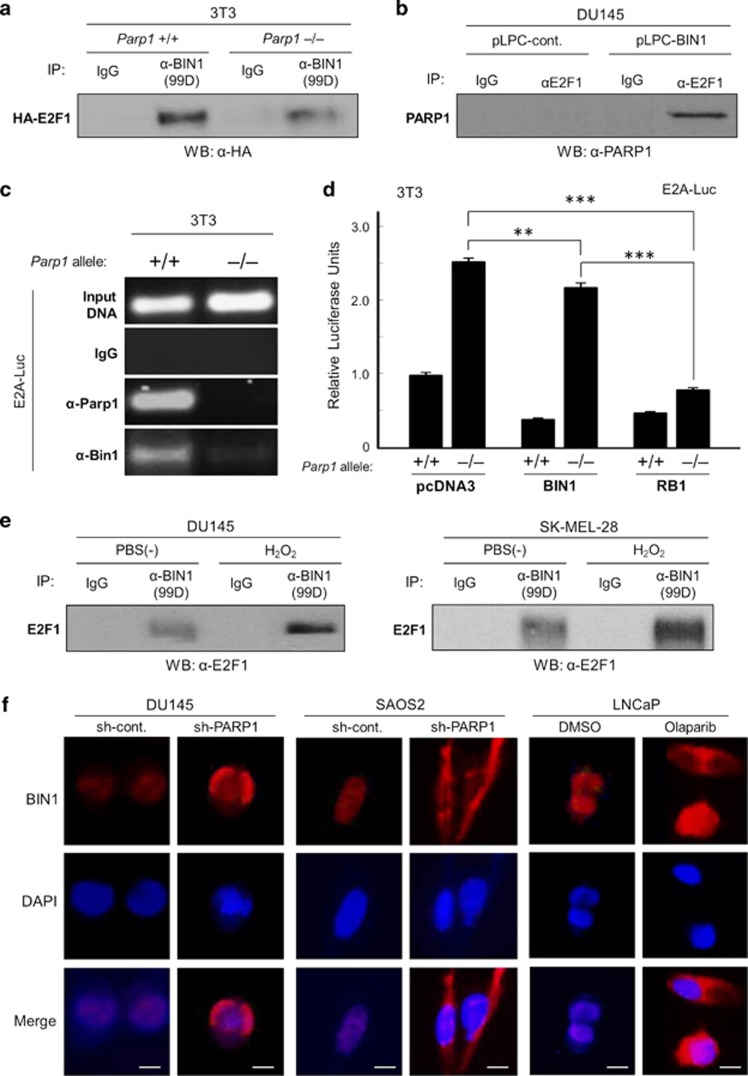
Figure 5.
PARP1 stabilizes the E2F1–BIN1 interaction in the nucleus. (a) Western blotting analysis of HA-tagged E2F1 protein (HA–E2F1) co-immunoprecipitated (co-IPed) with exogenously co-expressed BIN1 in the presence or absence of Parp1 in mouse 3T3 fibroblasts. (b) Western analysis of endogenous PARP1 protein co-IPed with endogenous E2F1 in the presence or absence of overexpressed BIN1 in DU145 cells. (c) Chromatin immunoprecipitation (ChIP) assay in mouse 3T3 Parp1+/+ and Parp1–/– fibroblast lines transiently transfected with E2A–Luc. Endogenous mouse Parp1 and Bin1 proteins were co-IPed with the E2A promoter. (d) E2A–Luc reporter assays in 3T3 Parp1+/+ and Parp1–/– fibroblast cells cotransfected with pcDNA3, BIN1, or RB1. ***P<0.0005. (e) Western blot analysis of endogenous E2F1 protein co-IPed with an anti-BIN1 (clone 99D) antibody after treatment with or without H2O2 (10 μM, 30 min) in two independent cell lines, DU145 (RB1-deficient) and SK-MEL-28 (RB1-proficient). (f) In situ immunofluorescence microscopy. Subcellular localization of endogenous BIN1 protein (red) was detected with an anti-BIN1 antibody (clone D3). DAPI was used for nuclear counterstaining (blue). Scale bar=10 μM