Figure 2.
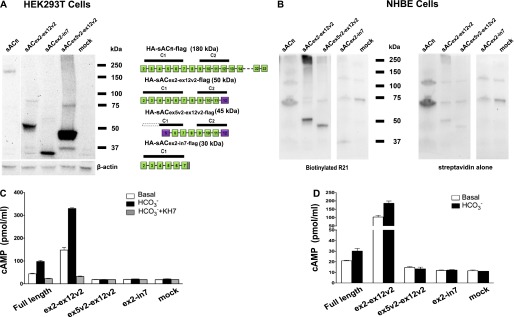
In vitro catalytic activity of recombinant sAC variants expressed in HEK293T and NHBE cells. (A) Lentivirus expression plasmids for sACfl and several variants shown on the right were transiently transfected into HEK293T cells. After 3 days, whole-cell protein lysates were prepared and equal amounts were separated via SDS-PAGE and blotted to a polyvinylidene difluoride membrane. The membrane was probed with anti–sAC R21. The blot was stripped and reprobed for β-actin as a loading control. (B) Undifferentiated NHBE cells were infected with lentiviruses, driving the expression of N-terminal hemagglutinin (HA)– and C-terminal flag–tagged human sACfl and several variants (shown on the left). Cells were redifferentiated using air–liquid interface (ALI) conditions. Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors and immunoprecipitated with an antibody to flag (cell lysates alone had insufficient sAC activity for analysis). Aliquots of the immunoprecipitated protein were run on SDS-PAGE, blotted to a nylon membrane, probed with a biotinylated anti–sAC R21 followed by horseradish peroxidase–streptavidin. The blot was stripped and reprobed with streptavidin to identify background streptavidin-binding proteins. (C) In vitro cyclase activity measured using a cAMP assay of cell lysates of transfected HEK293T cells. White bars show the basal level of activity; black bars show activity in the presence of 40 mM HCO3−, and gray bars show activity in the presence of 40 mM HCO3− plus 50 μM KH7. Activity was normalized to the expression levels of sAC variants using the Western blot data (A). Data are shown as mean (± SEM) from three experiments done as triplicates. (D) In vitro cyclase activity measured using a cAMP assay of infected NHBE after immunoprecipitation with mouse flag antibody–coated sepharose beads (cell lysates could not be used, because expression levels were insufficient to reliably measure cyclase activity). White bars show the basal level of activity and black bars show activity in the presence of 40 mM HCO3−. KH7 couldn’t be used because of interference with detergent. Activity was normalized to the expression levels of sAC variants using the Western blot data (B). Data are shown as mean (± SEM) from three experiments done as triplicates.
