Abstract
Carboplatin, a second-generation platinum chemotherapeutic drug, is considerably less ototoxic than cisplatin. While common laboratory species such as mice, guinea pigs and rats are highly resistant to carboplatin ototoxicity, the chinchilla stands out as highly susceptible. Moreover, carboplatin causes an unusual gradient of cell death in chinchillas. Moderate doses selectively damage type I spiral ganglion neurons (SGN) and inner hair cells (IHC) and the lesion tends to be relatively uniform along the length of the cochlea. Higher doses eventually damage outer hair cells (OHC), but the lesion follows the traditional gradient in which damage is more severe in the base than the apex. While carboplatin ototoxicity has been well documented in adult animals in vivo, little is known about its in vitro toxicity. To elucidate the ototoxic effects of carboplatin in vitro, we prepared cochlear and vestibular organotypic cultures from postnatal day 3 rats and adult chinchillas. Chinchilla cochlear and vestibular cultures were treated with carboplatin concentrations ranging from 50 µM to 10 mM for 48 h. Consistent with in vivo data, carboplatin selectively damaged IHC at low concentrations (50–100 µM). Surprisingly, IHC loss decreased at higher doses and IHC were intact at doses exceeding 500 µM. The mechanisms underlying this nonlinear response are unclear but could be related to a decrease in carboplatin uptake via active transport mechanisms (e.g., copper). Unlike the cochlea, the carboplatin dose-response function increased with dose with the highest dose destroying all chinchilla vestibular hair cells. Cochlear hair cells and auditory nerve fibers in rat cochlear organotypic cultures were unaffected by carboplatin concentrations <10 µM; however, the damage in OHC were more severe than IHC once the dose reached 100 µM. A dose at 500 µM destroyed all the cochlear hair cells, but hair cell loss decreased at high concentrations and nearly all the cochlear hair cells were present at the highest dose, 5 mM. Unlike the nonlinear dose-response seen with cochlear hair cells, rat auditory nerve fiber and spiral ganglion losses increased with doses above 50 µM with the highest dose destroying virtually all SGN. The remarkable species differences seen in vitro suggest that chinchilla IHC and type I SGN posse some unique biological mechanism that makes them especially vulnerable to carboplatin toxicity.
Keywords: Carboplatin, ototoxicity, organotypic culture
Introduction
Carboplatin (diammine [1,1 cyclobutane dicarboxylato (2)-0,0' ] platinum) is one of the platinum agents with enhanced anti-tumor activity and reduced side effects in comparison with cisplatin [1–6]. As a second-generation platinum compound, carboplatin expresses its anti-neo-plastic effect in a similar fashion as cisplatin by forming inter/intra-strand DNA cross-links when activated by conversion into aquated species [7, 8]. For the reason of being highly efficient in anti-cancer actions and of low toxic side-effects, carboplatin has been used widely to treat various types of solid tumors in humans. The side effects of carboplatin were similar to all adverse effects of cisplatin, such as myelosuppression, nephrotoxicity, gastrointestinal upset, peripheral neurotoxicity, electrolyte disturbance and hepatotoxicity, as well as ototoxicity.Although the level of side effects of carboplatin is less severe than that of cisplatin, however, they have attracted increasing attention from clinical doctors and research scientists[9–22].
The toxic effects of carboplatin were discussed in clinic reports in as early as the 80s [23–28]. Since then, the ototoxicity of carboplatin has been studied in several common laboratory species, such as rats [29–35]. guinea pigs [6, 30, 36–40], rabbits [41], monkeys [42], mice [43–48], and zebrafish [49]. The ototoxic effects of carboplatin have been observed vary significantly across these species. There is very little evidence of ototoxicity in mice. Guinea pigs are relatively resistant to carboplatin, but damage can occur when extremely high doses of carboplatin are given. High doses of carboplatin can cause high frequency hearing loss and outer hair cell lesion in guinea pigs and the damage progresses from the base of the cochlea to the apex, much like most other ototoxic drugs, such as aminoglycoside antibiotics, or cisplatin [50–53]. In laboratory rats, moderate-doses of carboplatin do not affect the cochlea[54]. However, high doses of carboplatin result in a significant reduction in amplitude of distortion product otoacoustic emissions (DPOAEs) which suggests that the severe damage takes place at the outer hair cells level [34, 55]. In contrast to all the above mentioned animals, chinchillas treated with carboplatin develop an unusual hair cell lesion that selectively destroys the inner hair cells and type I spiral ganglion neurons in the cochlea [10,56–74]. In the chinchilla vestibular system, the ototoxic effects of carboplatin has been studied, showing that Type I hair cells and larger ganglion neurons in the vestibular system are more susceptible to carboplatin [60, 66, 71, 74, 75]. Although the ototoxic effects of carboplatin have been well documented in various experimental animal species, however, the mechanism of carboplatin ototoxicity is not quite clear. Specifically, the different targeting effects of carboplatin in the cochlea among the chinchilla and other species are unknown.
The in vitro ototoxic effects of carboplatin have been studied in very few species using as cell lines and cochlear cultures [30, 76–78]. According to the very limited discoveries from previous studies in the rat cochlear culture system, carboplatin absolutely selectively destroys outer hair cells and spiral ganglion neurons in the postnatal day 3 rat cochlear explant [76–78]. In contrast, carboplatin selectively destroys inner hair cells and type I spiral ganglion neurons in chinchilla cochlear explants as it does in vivo, from our preliminary findings reported in an abstract at the Association for Research in Otolaryngology annual meeting[77]. To determine if the striking species differences in carboplatin ototoxicity are due to cellular intrinsic response, we evaluated dose dependent cell degeneration using cochlear and vestibular organotypic cultures treated with various concentrations of carboplatin from postnatal day 3 rat pups, adult rats, and adult chinchillas respectively.
Methods
Cochlear and vestibular organotypic cultures and carboplatin treatment
The cochlear and vestibular organ culture procedures are similar to those described previously [50,79–85]. The organotypic cultures of cochlear and vestibular end-organs were prepared from postnatal day 3 SASCO Sprague-Dawley rats, adult SASCO Sprague-Dawley rats, and adult chinchillas respectively. Experiments were performed according to the rules and regulations of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and the National Institutes of Health Guidelines for the Care and Use of LaboratoryAnimals.
For culturing cochlear and vestibular explants from postnatal day 3 rats, a drop of rat tail type I collagen gel was added in Basal Medium Eagle containing 2% sodium carbonate in a 35 mm culture dish. Type I rat-tail collagen (Collaborative Research, 3.76 mg/ml in 0.02 N acetic acid) was mixed with 10X Basal Medium Eagle (BME, Sigma) and 2% sodium carbonate at a 9:1:1 ratio. A 10 µl drop of the collagen solution was placed on the surface of a 35 mm culture dish and allowed to gel for approximately 30 min. Afterwards, 1.3 ml of culture medium (0.01 g/ml bovine serum albumin, 1% Serum-Free Supplement [Sigma I-1884], 2.4% of 20% glucose, 0.2% penicillin G, 1% BSA, 2 mM glutamine, 95.4% of 1X BME) was added to the dish to level the apical of the collagen gel. The cochlear basilar membrane including spiral ganglion neurons in Rosenthal's canal and vestibular end-organs including the maculae of saccule and utricle, and cristae of ampulla were carefully micro-dissected out, and positioned on the drop surface of collagen gel and a flat surface preparation was made by gently pressing on the tissue with forceps. Surface tension from the thin layer of culture medium helped to hold the tissue against the underlying collagen. The cochlear and vestibular explants were placed in an incubator (Forma Scientific 3029, 37°, 5% CO2) overnight. On the second day, the serum-free medium was exchanged with new medium that contained a specific concentration of carboplatin (10 µM, 50 µM, 100 µM, 500 µM, 1000 µM, 5000 µM, or 10000 µM, Sigma C2538), and incubated for 48 h. Control samples containing only the serum-free medium, were run concurrently with the experimental samples.
For culturing cochlear and vestibular explants from adult rats and chinchillas, the cochlear basilar membrane and vestibular end-organs including the maculae of saccule and utricle, and crista of ampulla were micro-dissected out and embedded in the fresh-made rat-tail collagen gel. After the collagen solution became the gel, 2 ml of serum-free culture medium was added to the dish for overnight incubation (Forma Scientific 3029, 37°, 5% CO2). The serum-free medium containing a specific concentration of carboplatin (50 µM, 100 µM, 500 µM, 1000 µM, 5000 µM, or 10000 µM, Sigma C2538) was added on the second day for another 48 h incubations.
Histology
At the end of the experiment, the cochlear and vestibular explants were fixed for 2 h with4% formalin in 0.1Mphosphate buffer (pH 7.4). Specimens from postnatal day 3 rat pups were double-labeled with a monoclonal antibody against neurofilament 200 (Sigma N0142, clone N52) to show the auditory nerve fibers and spiral ganglion neurons plus phalloidin conjugated Alexa Fluor 488 (Invitrogen A12379) to label the cuticular plate and stereocilia bundles of the hair cells. After double labeling, specimens were rinsed in PBS, and then immersed overnight (4³C) in solution containing 20 µl of mouse anti-neurofilament 200 antibody (Sigma p1951, 1:100) dissolved in a solution containing 20 µl Triton X-100 (10%), 6 µl normal goat serum, 154 µl of 0.1 MPBS. After rinsing in 0.1 MPBS and immersed in a solution containing 20 µl of secondary goat anti-mouse IgG TRITC (Sigma T5393, 1:200) mixed in 12 µl normal goat serum, 40 µl Triton X-100 (10%) and 328 µl of 0.1 M PBS. Specimens were rinsed three times in PBS, and then stained with Alexa Fluor 488 conjugated phalloidin (1:200) for 30 min. After rinsing with PBS, specimens were mounted on glass slides as surface preparations in glycerin. Specimens from adult chinchillas were just stained with Alexa Fluor 488 conjugated phalloidin or TRITC-labeled phalloidin (Sigma P1951,1:200) for 30 min, and mounted on glass slides in glycerin. Samples were examined using a confocal microscope (Zeiss LSM-510 meta, step size 0.5 µm per slice with appropriate filters to detect the red fluorescence of TRITC labeled neurofilament 200 in nerve fibers and spiral ganglion neurons or TRITC labeled phalloidin at F-actin in chinchilla stereocilia (excitation 544 nm, emission 572 nm) and green fluorescence of Alexa 488-labeled phalloidin (excitation 488 nm, emission 520 nm) in the cuticular plate and the stereocilia of the hair cells. Confocal images from multiple layers were projected onto a single plane using the Zeiss LSM Image Examiner (version: 4, 0, 0, 91). Confocal images were further processed using adobe photoshop 5.5 software.
Results
Damage in cochlear explants from postnatal day 3 rat pups
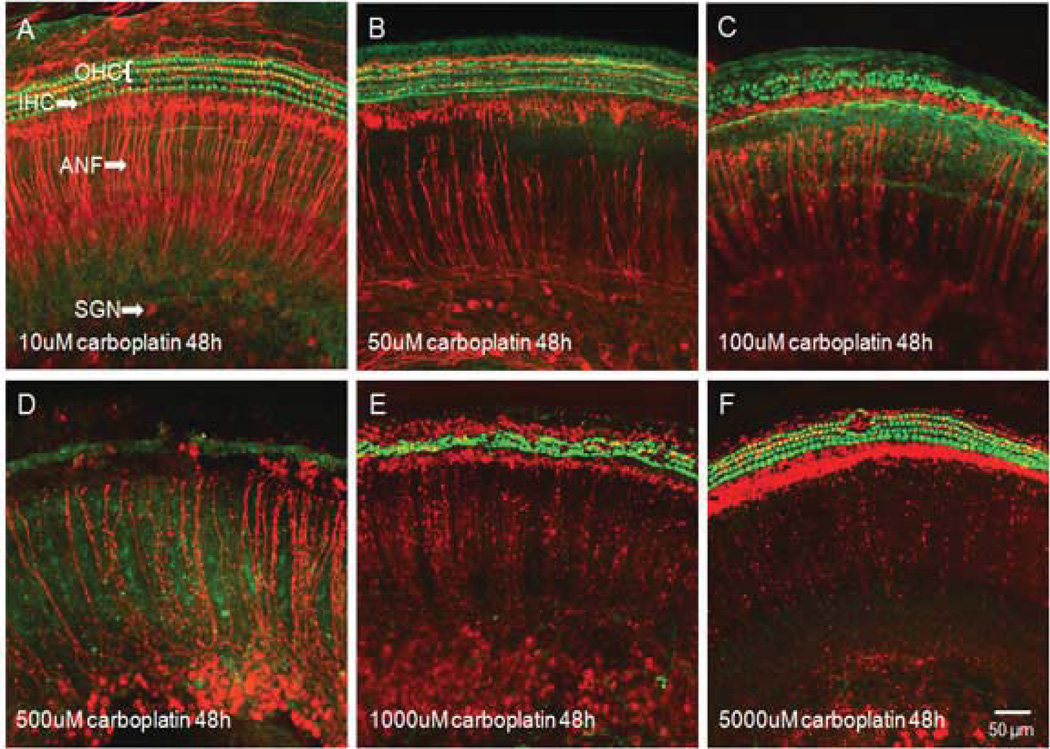
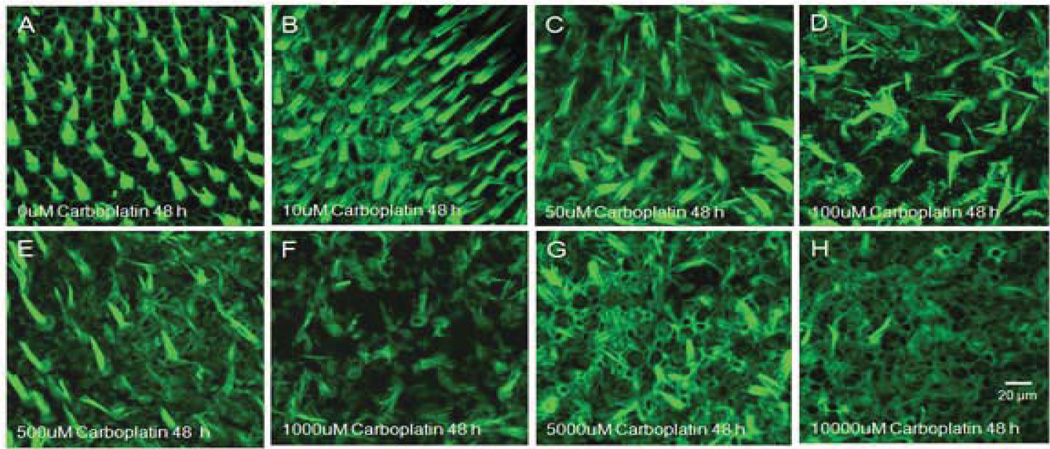
In the cochlear explants from postnatal day 3 rat pups, carboplatin treatment for 48 h at various concentrations (10 µM, 50 µM, 100 µM, 500 µM, 1000 µM, or 5000 µM) resulted in two distinct patterns of damage of the cochlear hair cells and the spiral ganglion neurons (Fig. 1). Cochlear hair cells were heavily labeled with Alexa 488 phalloidin (green) and the auditory nerve fibers and spiral ganglion neurons were stained with neurofilament 200 antibody (red) respectively in normal cochlear explants after 48 h of culturing in standard serum-free medium without carboplatin (Fig. 1A). After treatment with 50 µM carboplatin for the same duration, cochlear hair cells were intact, while the density of auditory nerve fibers started to decline (Fig. 1B). When carboplatin concentration increased to 100 µM, the damage to hair cell and auditory nerve fibers were more evident (Fig. 1C). Carboplatin treatment at 500 µM destroyed all hair cells and heavily damaged auditory nerve fibers and spiral ganglion neurons (Fig. 1D). It is worthwhile to note that when carboplatin concentration was increased to 1000 µM, the damage to cochlear hair cells actually decreased, although degeneration of auditory nerve fibers and spiral ganglion neurons became worse (Fig. 1E). One interesting phenomenon is that 5000 µM carboplatin resulted in a complete destruction to auditory nerve fibers and spiral ganglion neurons, but most cochlear hair cells survived (Fig. 1F).
Figure 1.
Photomicrographs of representative cochlear organotypic cultures double labeled with a monoclonal antibody against neurofilament 200 to show the auditory nervous system (red) and phalloidin to show the stereocilia bundles of the hair cell (green). (A) A cochlear explant treated with 10 µM carboplatin for 48 h showing no obvious damage of either hair cells or auditory nervous system. (B) A specimen treated with 50 µM carboplatin showing substantial damage of auditory nerve fibers. (C) A specimen treated with 100 µM carboplatin showing massive loss of both auditory nerve fibers and cochlear hair cells. (D) A specimen treated with 500 µM carboplatin showing destruction of most hair cells and severe damage to the auditory nervous system. (E) Even greater auditory nervous degeneration after 1000 µM carboplatin treatment, although with less missing hair cells. (F) Complete destruction of auditory nerve fibers and spiral ganglion neurons following 5000 µM carboplatin treatment. Unexpectedly, most cochlear hair cells have survived.
Hair cell damage in vestibular explants from postnatal day 3 rat pups
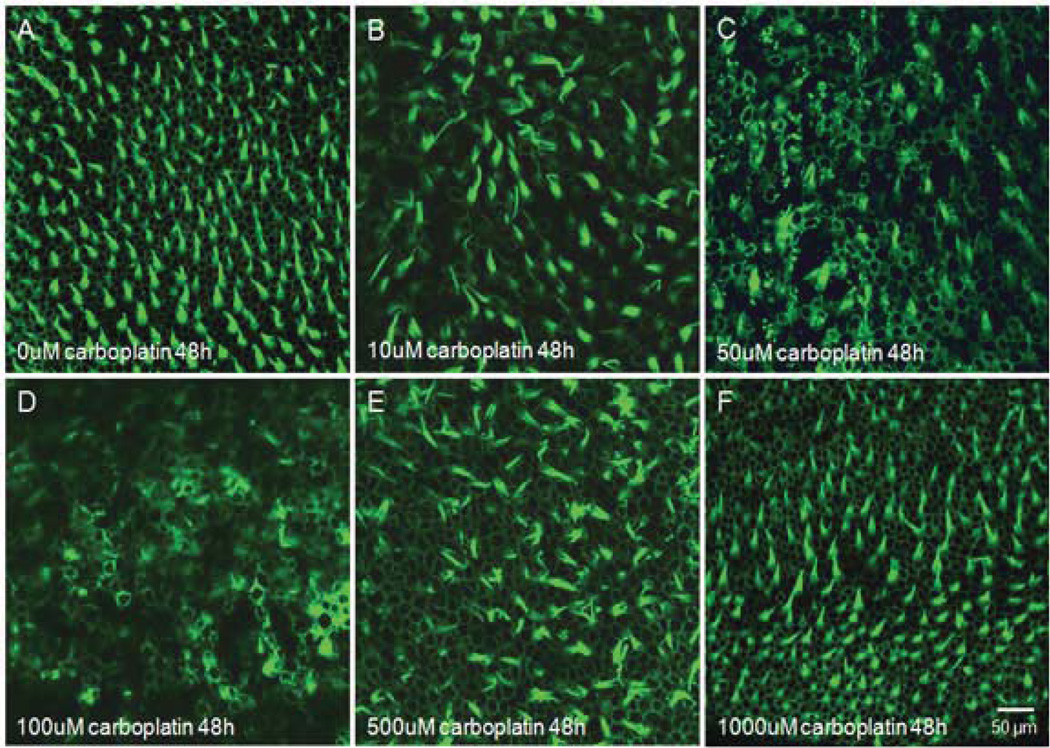
The photomicrographs in figure 2 are representatives of macula of utricle 48 h after culturing with various concentrations of carboplatin. Figure 2A shows normal surface structures of vestibular hair cells from a normal, untreated macula of utricle. Exposing vestibular explants to 10 µM carboplatin for 48 h resulted in a reduction of hair cell density (Fig. 2B). When the concentration of carboplatin increased to 50 µM or 100 µM, most hair cells were completely destroyed (Fig. 2C, 2D). However, as can be seen in figure 2E and 2F, the hair cell survival was distinctly increased when the concentration of carboplatin exceeded 500 µM.
Figure 2.
Photomicrographs of rat vestibular hair cells in the macula of utricles labeled with Alexa Fluor 488 conjugated phalloidin. (A) Untreated vestibular explants after 48 h culturing, showing well-organized vestibular hair cells as in normal controls. (B) Visible hair cell loss after treatment with 10 µM carboplatin. (C) Massive loss of vestibular hair cells after 50 µM carboplatin treatment. (D) Destruction of most vestibular hair cells after treatment with 100 µM carboplatin. (E) Increased hair cells survival after culturing with 500 µM carboplatin. (F) Mostly intact hair cells after 1000 µM carboplatin treatment.
Hair cell damage in cochlear explants from adult rats
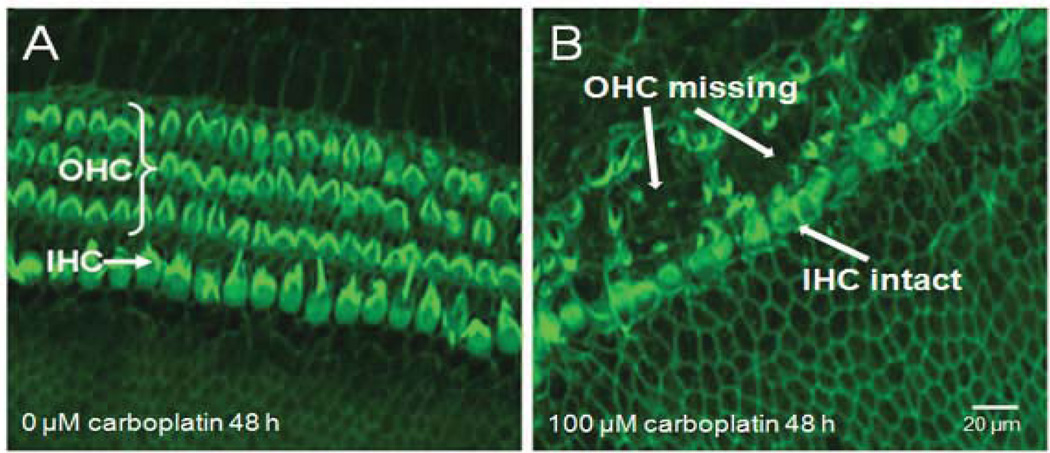
The dose effects of carboplatin on adult rat cochlear explants were somewhat similar to those on postnatal day 3 rat pups explants: i.e., low dose carboplatin damaged hair cells while high concentration of carboplatin did not. In addition, outer hair cells were clearly damaged more than inner hair cells (Fig. 3), consistent with previous in vivo and in vitro studies in rats [31, 34, 76–78].
Figure 3.
Carboplatin-induced structural damage on the organ of Corti in adult rat cochlear explants. (A) normal control rat cochlear orgnotypic culture in standard serum-free medium after 48 h without carboplatin treatment. (B) Rat cochlear explants treated with 100 µM carboplatin for 48 h. Note massive loss of outer hair cells, with essentially intact inner hair cells.
Hair cell damage in vastibular explants from adult rats
A similar destructive trend to vestibular explants from postnatal day 3 rat pups by carboplatin was found in adult rat vestibular culture system. Examination of the vestibular hair cells following 48 h of carboplatin exposure at various concentrations (10 µM, 50 µM, 100 µM, 500 µM, 1000 µM, or 5000 µM) revealed that vestibular hair cells were destroyed at lower concentrations (10 µM, 50 µM, 100 µM, and 500 µM) (Fig. 4A, 4B, 4C, and 4D), but remained intact at the higher concentrations (1000 µM, and 5000 µM) (Fig. 4E, and 4F).
Figure 4.
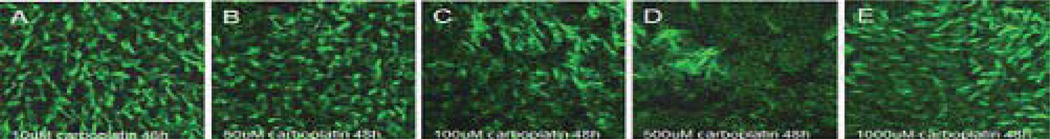
Photomicrographs of rat vestibular hair cells in the macula of utricles labeled with Alexa Fluor 488 conjugated phalloidin. (A) Most hair cells were visible 48 h after 10 µM carboplatin treatment. (B) Tangling and breaking cilia were detected 48 h post-50 µM carboplatin. (C) Visible damage on the cuticular plate of hair cells was found 48 h after 100 µM carboplatin cultures. (D) 50 µM carboplatin treatment for 48 h resulted in massive destruction of hair cells. (E) Most hair cells were intact 48 h after 1000 µM carboplatin treatment.
Hair cell damage in cochlear explants from adult chinchillas
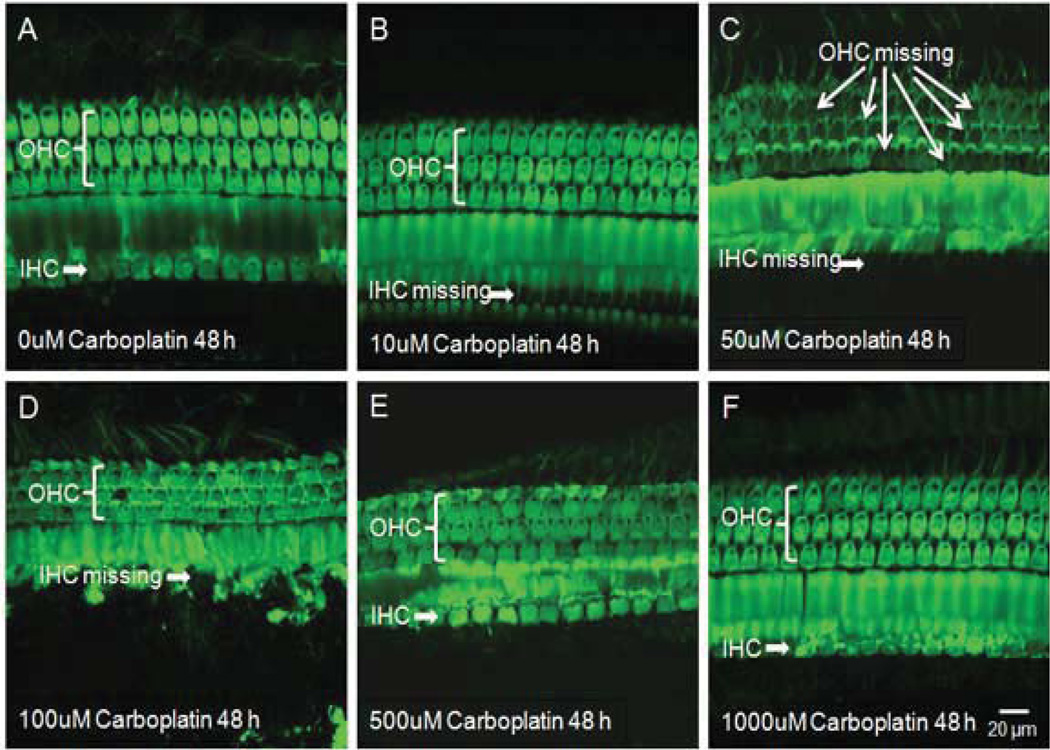
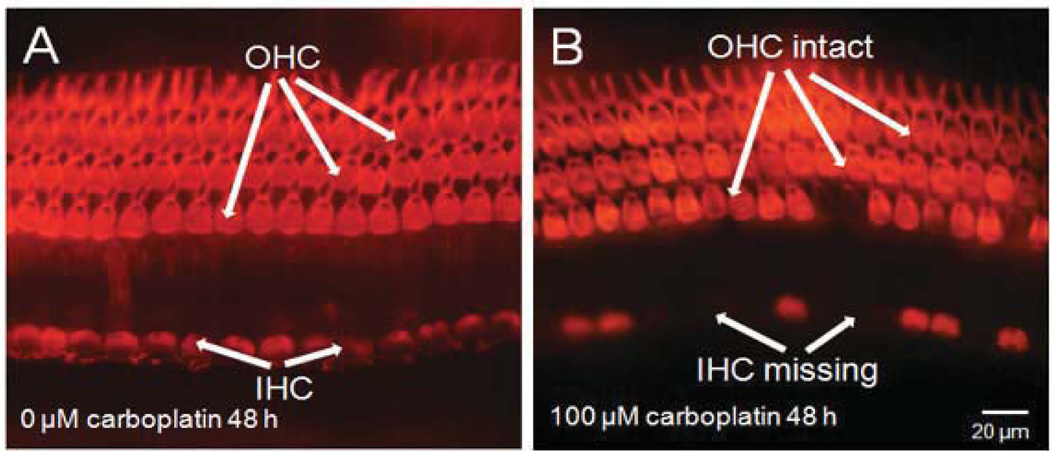
The photomicrograph in Fig. 5A shows the orderly structure of three rows of outer hair cells and a single row of inner hair cells in a normal cochlear organotypic culture from an adult chinchilla. carboplatin treatment at 10 µM for 48 h resulted in the loss of inner hair cells, while sparing the outer hair cells (Fig. 5B). When the dose of carboplatin was increased to 50 µM, besides the IHC loss, most outer hair cells were also destroyed (Fig. 5C). However, exposure to 100 µM carboplatin for 48 h only destroyed the inner hair cells, but not the outer hair cells (Fig. 5D). Surprisingly, when the concentration of carboplatin amounted to over 500 µM, most inner hair cells and outer hair cells were intact (Fig. 5E, 5F). Severe hair cell loss was detected following 48 h exposure to 50 µM carboplatin, whereas no striking damage to hair cells were seen after exposure to 500 µM or higher concentrations of carboplatin. Figure 6 shows the selective damage to inner hair cells in chinchilla cochlear explants, concordant with in vivo observation of carboplatin-induced inner hair cell degeneration in chinchillas[12, 56–59, 61–64, 73, 86, 87].
Figure 5.
Surface preparations showing the dose response of carboplatin in cochlear organotypic cultures from adult chinchillas. (A) All inner and outer hair cells are intact 48 h after culturing with standard culture medium without carboplatin. (B) Massive inner hair cell loss after 10 µM carboplatin treatment for 48 h while outer hair cells are spared. (C) Destruction of both inner and outer hair cells after treatment with 50 µM carboplatin for 48 h (D), Missing inner hair cells with obvious damage in their surrounding supporting cells after 48 h culturing with 100 µM carboplatin. However, most outer hair cells have survived. (E) With the concentration of carboplatin increased to 500 µM, most inner and outer hair cells remain essentially normal. (F) Carboplatin treatment at 1000 µM for 48 h results in no damage in hair cells.
Figure 6.
Carboplatin-induced structural damage on the organ of Corti in chinchilla cochlear explants. (A) Normal cochlear hair cells after 48 h culturing without carboplatin. (B) Chinchilla cochlear explants treated with 100 µM carboplatin for 48 h. Note many missing inner hair cells, but most outer hair cells remain normal.
Hair cell damage in vestibular explants from adult chinchillas
Exposing vestibular explants from adult chinchillas to carboplatin with various concentrations (10 µM, 50 µM, 100 µM, 500 µM, 1000 µM, or 10000 µM) for 48 h resulted in the destruction of hair cells in macular of utricle in a dose-dependent fashion (Fig. 7). The photomicrograph in Figure 7A shows the normal vestibular culture labeled with Alexa Fluor 488-conjugated phalloidin that intensely labels actin in the stereocilia and, to a lesser extent, the cuticular plate of hair cells. As can be seen in figure 7B and figure 7C, the low doses of carboplatin used (10 µM and 50 µM) did not cause evident damage to vestibular hair cells. However, carboplatin at 100 µM was sufficient to bring about the disarray of stereocilia bundles on some vestibular hair cells (Fig. 7D). When the concentration of carboplatin was increased to 500 µM, cilia missing was seen in many vestibular hair cells leaving remnants of the actin ring surrounding the cuticular plate (Fig. 7E). Increasing the carboplatin dose to 1000, 5000, or 10000 µM destroyed most vestibular hair cells (Fig. 7F, 7G, 7H). These results indicate that carboplatin-induced vestibular hair cell degeneration is in a dose-dependent manner in vestibular culture system from adult chinchilla.
Figure 7.
Photomicrographs showing vestibular organotypic cultures from the middle area of macula of utricle after 48 h treatment with carboplatin in an adult chinchilla culture system. Hair cells are labeled with Alexa Fluor 488-phalloidin. Carboplatin concentrations are shown in each panel. (A) A normal control showing normal arrangement of vestibular hair cells. Vestibular culture treated with carboplatin at a concentration of 10 µM shows no damage of hair cells (B). However, carboplatin treatment at 50 µM and 100 µM for 48 h results in abnormal arrangement of vestibular hair cells in the macula of utricle (C, D). Cilia in some vestibular hair cells missing after 48 h of carboplatin treatment at 500 µM. (E). Massive damage in the macula of utricle when the concentrations of carboplatin exceeds 1000 µM (F, G, H).
Discussion
As a second-generation platinum antineoplastic agent, carboplatin is about the same as cisplatin in biological effects due to formation of the same interstrand/intrastrand DNA cross links. Although the incidence of carboplatin side effects is reduced, however, the antitumor potential of carboplatin remains the same as its original design thanks to technological innovation[2]. Despite that carboplatin has a lower risk of producing adverse reactions than cisplatin, there has been a growing concern over its adverse neurotoxic and ototoxic potentials in recent years[10,14,15]. In different experimental animal species, carboplatin has been documented as predominantly affecting outer hair cells in rats and guinea pigs[34, 37, 89]. In contrast to the findings in these species, carboplatin-induced selective inner hair cell damage in chinchillas becomes a unique, species-specific toxicity[56, 59, 61–63, 65–67, 74, 75, 82]. An intriguing question that needs to be addressed is why the various experimental animal species have different responses to carboplatin ototoxicity. There has not been a definite answer. Generally, different drug-induced responses in different species are considered to be related to either species diversity or the cell difference. To comprehend if the difference in susceptibility to carboplatin ototoxicity in different species is determined by the cell itself rather than the systemic drug metabolism in living animal species, we compared the in vitro carboplatin ototoxic effects between chinchillas and rats. According to the results from inner ear organotypic cultures in the current study, the species-specific ototoxicity of carboplatin is believed to be characterized at the cell level rather than the systemic metabolism.
The biological activation of carboplatin requires hydration following cell entry. In order to bind to DNA and to exert its toxic effects, carboplatin must first enter the cell. Evidence suggests that platinum agents are mainly imported by copper transporter importer, Ctr1, and exported from cells or cellular organelles by copper transporter exporters, ATP7A, and ATP7B[74, 82, 84, 85, 90–93, 102]. Cells have the potential to adjust the activities of copper transporters properly according to the intracellular and extracellular copper/platinum concentrations[82, 84, 85, 95–103]. According to reports in the literature, enhanced extracellular concentration of copper by local application on round window can result in an intrinsic cytoprotective effect of hair cells against carboplatin by modulating the activities of copper transporters[53, 82, 84, 85, 102]. These important findings suggest that intracellular and extracellular copper/platinum is detectable by the cells. Experiments indicate that the high concentration of copper/platinum can lead to a reduction of uptake by withdrawing Ctr1 from the membrane into the cytoplasm where Ctr1 is quickly degraded, and also increased efflux of copper/platinum from cytoplasm by ATP7A and ATP7B to reduce the accumulation of copper/platinum in the cell[53, 82, 84, 85, 102]. The cellular intrinsic modulation of copper transporters is efficient for the cytoprotection from platinum injury in vitro, but may not appear in vivo natively. The cause is that in vitro concentrations of platinum can be high enough to alert the cell. However, the in vivo concentration of platinum in the inner ear is low due to the block by the barrier between the bloodstream and organic tissues[52, 102, 104–107]. Therefore, the infiltration of platinum in the inner ear by systemic absorption may not attain enough concentration to attract cell’ s attention. An interesting phenomenon discovered is that cochlear and vestibular hair cells from postnatal day 3 rats and adult rats showed a similar nonlinear response to carboplatin ototoxicity, while the damage to auditory nerve fibers and spiral ganglion neurons in postnatal day 3 rats was in a linear dose dependent manner. This is different from neurotoxic effects of cisplatin in rat cochlear organotypic cultures[82, 84, 85]. In comparison with rat cochlear organotypic cultures, cochlear hair cells of chinchilla were destroyed by carboplatin only at low concentrations, which is similar to the response from in vitro studies in various platinum explants[82–85, 102, 108]. The only difference is that inner hair cells in chinchilla are more susceptible to carboplatin, while outer hair cells in rat are the first victims. The species-specific mechanism of this phenomenon is unclear, but will need to be addressed in our future studies. The rat vestibular hair cells have equal dose-response to carboplatin in that these cells are destroyed by low concentration of carboplatin treatment, but remain intact at high concentrations. However, chinchilla vestibular hair cells exhibited a dose dependent response to carboplatin. The cause of the differences between rats and chinchillas in responses to carboplatin ototoxicity in vestibular hair cells is not known. A protective response may be aroused in rat vestibular hair cells by high concentrations of platinum[82, 85, 102, 105]. In contrast, the vestibular hair cells in chinchilla do not show this intrinsic resistance to carboplatin for some unknown reasons[76, 77].
To prevent the ototoxic effects of platinum, many strategies can be considered for inner ear protection. Since platinum can arouse cellular injury by activation of free radicals[76, 109–113], many antioxidants have been used for protection against cisplatin toxicity[112, 114–118]. Although antioxidation can effectively protect the cochlea from platinum damage, it can also compromise the anti-tumor activity of platinum when treated systemically[119,120]. Therefore,local antioxidant application may be required for cochlear protection against platinum ototoxicity [118]. Platinum toxicity specifically causes cell apoptosis. Therefore, anti-apoptotic agents may also provide protection against platinum toxicity[82,85,121–139]. In addition, neurotrophic factors have also been reported to reverse platinum induced cochlear injury[140–142]. Although above mentioned strategies have seen variable successes, it has to be pointed out that the key is the process of cell degeneration. When the cell has started the degeneration process, treatment effects will vary depending on the degree of lesions. Therefore, the protective actions by antioxidation or anti-apoptosis agents are limited. The results in our recent publications and the current study, by modulating copper transporters, may develop a new strategy against platinum ototoxicity, which keeps cisplatin out of the cell by reducing platinum influx and enhancing platinum efflux[50, 53, 74, 82–85, 102, 105, 108]. When the platinum is isolated from the cell while in the process of attack, its following toxic effects may be unfulfilled.
References
- 1.Calvert AH, Harland SJ, Mewell DR, et al. Phase I studies with carboplatin at the Royal Marsden Hospital. Cancer Treat Rev. 1985;12(Suppl A):51–57. doi: 10.1016/0305-7372(85)90018-0. [DOI] [PubMed] [Google Scholar]
- 2.Calvert AH, Harland SJ, Newell DR, et al. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol. 1982;9(3):140–147. doi: 10.1007/BF00257742. [DOI] [PubMed] [Google Scholar]
- 3.Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984;44(4):1693–1697. [PubMed] [Google Scholar]
- 4.Woloschuk DM, Pruemer JM, Cluxton RJ. Carboplatin: a new cisplatin analog. Drug Intell Clin Pharm. 1988;22(11):843–849. doi: 10.1177/106002808802201101. [DOI] [PubMed] [Google Scholar]
- 5.McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995;13(4):228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe KC, Jinnouchi K, Hess A, et al. Carboplatin induces less apoptosis in the cochlea of guinea pigs than cisplatin. Chemotherapy. 2002;48(2):82–87. doi: 10.1159/000057667. [DOI] [PubMed] [Google Scholar]
- 7.Canetta R. The development and characteristics of carboplatin, a second generation platinum compound. Lectures and Symposia 14th Int. Cancer Congress, Budapest. 1986;9:19–26. [Google Scholar]
- 8.van Hennik MB, van der Vijgh WJ, Klein I, et al. Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res. 1987;47(23):6297–6301. [PubMed] [Google Scholar]
- 9.Kennedy IC, Fitzharris BM, Colls BM, et al. Carboplatin is ototoxic. Cancer Chemother Pharmacol. 1990;26(3):232–234. doi: 10.1007/BF02897206. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald MR, Harrison RV, Wake M, et al. Ototoxicity of carboplatin: comparing animal and clinical models at the Hospital for Sick Children. J Otolaryngol. 1994;23(3):151–159. [PubMed] [Google Scholar]
- 11.Parsons SK, Neault MW, Lehmann LE, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22(7):669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 12.Lockwood DS, Ding DL, Wang J, et al. D-Methionine attenuates inner hair cell loss in carboplatin-treated chinchillas. Audiol Neurootol. 2000;5(5):263–266. doi: 10.1159/000013890. [DOI] [PubMed] [Google Scholar]
- 13.Montaguti M, Brandolini C, Ferri GG, et al. Cisplatin and carboplatin-induced ototoxicity in children: clinical aspects and perspectives for prevention. Acta Otorhinolaryngol Ital. 2002;22(1):14–18. [PubMed] [Google Scholar]
- 14.Simon T, Hero B, Dupuis W, et al. The incidence of hearing impairment after successful treatment of neuroblastoma. Klin Padiatr. 2002;214(4):149–152. doi: 10.1055/s-2002-33179. [DOI] [PubMed] [Google Scholar]
- 15.Dittrich Ch, Sevelda P, Salzer H, et al. Lack of impact of platinum dose intensity on the outcome of ovarian cancer patients. 10-year results of a prospective randomised phase III study comparing carboplatin-cisplatin with cyclophosphamide-cisplatin. Eur J Cancer. 2003;39(8):1129–1140. doi: 10.1016/s0959-8049(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 16.Kanat O, Evrensel T, Baran I, et al. Protective effect of amifostine against toxicity of paclitaxel and carboplatin in non-small cell lung cancer: a single center randomized study. Med Oncol. 2003;20(3):237–245. doi: 10.1385/MO:20:3:237. [DOI] [PubMed] [Google Scholar]
- 17.Smits C, Swen SJ, Theo Goverts S, et al. Assessment of hearing in very young children receiving carboplatin for retinoblastoma. Eur J Cancer. 2006;42(4):492–500. doi: 10.1016/j.ejca.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Dean JB, Hayashi SS, Albert CM, et al. Hearing loss in pediatric oncology patients receiving carboplatin-containing regimens. J Pediatr Hematol Oncol. 2008;30(2):130–134. doi: 10.1097/MPH.0b013e31815d1d83. [DOI] [PubMed] [Google Scholar]
- 19.Fouladi M, Gururangan S, Moghrabi A, et al. Carboplatin-based primary chemotherapy for infants and young children with CNS tumors. Cancer. 2009;115(14):3243–3253. doi: 10.1002/cncr.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal S, Merchant T, Reymond R, et al. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010;125(4):e938–e950. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musial-Bright L, Fengler R, Henze G, et al. Carboplatin and ototoxicity: hearing loss rates among survivors of childhood me-dulloblastoma. Childs Nerv Syst. 2011;27(3):407–413. doi: 10.1007/s00381-010-1300-1. [DOI] [PubMed] [Google Scholar]
- 22.Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30(10):1034–1041. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tange RA, Dreschler WA, van der Hulst RJ. The importance of high-tone audiometry in monitoring for ototoxicity. Arch Otorhinolaryngol. 1985;242(1):77–81. doi: 10.1007/BF00464411. [DOI] [PubMed] [Google Scholar]
- 24.Forastiere AA, Takasugi BJ, Baker SR, et al. High-dose cisplatin in advanced head and neck cancer. Cancer Chemother Pharmacol. 1987;19(2):155–158. doi: 10.1007/BF00254569. [DOI] [PubMed] [Google Scholar]
- 25.Gore ME, Calvert AH, Smith LE. High dose carboplatin in the treatment of lung cancer and mesothelioma: a phase I dose escalation study. Eur J Cancer Clin Oncol. 1987;23(9):1391–1397. doi: 10.1016/0277-5379(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 26.van der Hulst RJ, Dreschler WA, Urbanus NA. High frequency audiometry in prospective clinical research of ototoxicity due to platinum derivatives. Ann Otol Rhinol Laryngol. 1988;97(2 Pt 1):133–137. doi: 10.1177/000348948809700208. [DOI] [PubMed] [Google Scholar]
- 27.Shea TC, Flaherty M, Elias A, et al. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol. 1989;7(5):651–661. doi: 10.1200/JCO.1989.7.5.651. [DOI] [PubMed] [Google Scholar]
- 28.Wagstaff AJ, Ward A, Benfield P, et al. Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs. 1989;37(2):162–190. doi: 10.2165/00003495-198937020-00005. [DOI] [PubMed] [Google Scholar]
- 29.Kimura S, Nakajima Y, Hasegawa S, et al. Study on nephrotoxicity in rats receiving cis-diammine-1,1-cyclobutane dicarboxylate platinum II--special reference to morphological changes. Nihon Hinyokika Gakkai Zasshi. 1989;80(4):517–525. doi: 10.5980/jpnjurol1989.80.517. [DOI] [PubMed] [Google Scholar]
- 30.Neuwelt EA, Brummett RE, Remsen LG, et al. In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res. 1996;56(4):706–709. [PubMed] [Google Scholar]
- 31.Husain K, Whitworth C, Somani SM, et al. Carboplatin-induced oxidative stress in rat cochlea. Hear Res. 2001;159(1–2):14–22. doi: 10.1016/s0378-5955(01)00306-9. [DOI] [PubMed] [Google Scholar]
- 32.Emerich DF, Winn SR, Bartus RT. Injection of chemotherapeutic microspheres and glioma. IV: Eradicating tumors in rats. Cell Transplant. 2002;11(1):47–54. [PubMed] [Google Scholar]
- 33.Husain K, Whitworth C, Hazelrigg S, et al. Carboplatin-induced oxidative injury in rat inferior colliculus. Int J Toxicol. 2003;22(5):335–342. doi: 10.1177/109158180302200502. [DOI] [PubMed] [Google Scholar]
- 34.Okur E, Kilinc M, Yildirim I, et al. Effect of N-acetylcysteine on carboplatin-induced ototoxicity and nitric oxide levels in a rat model. Laryngoscope. 2007;117(12):2183–2186. doi: 10.1097/MLG.0b013e31813e6041. [DOI] [PubMed] [Google Scholar]
- 35.Esteban-Fernandez D, Verdaguer JM, Ramirez-Camacho R, et al. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J Anal Toxicol. 2008;32(2):140–146. doi: 10.1093/jat/32.2.140. [DOI] [PubMed] [Google Scholar]
- 36.Schweitzer VG, Rarey KE, Dolan DF, et al. Ototoxicity of cisplatin vs. platinum analogs CBDCA (JM-8) and CHIP (JM-9) Otolaryngol Head Neck Surg. 1986;94(4):458–470. doi: 10.1177/019459988609400410. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Saito H, Saito K, et al. Ototoxicity of carboplatin in guinea pigs. Auris Nasus Larynx. 1989;16(1):13–21. doi: 10.1016/s0385-8146(89)80003-3. [DOI] [PubMed] [Google Scholar]
- 38.Taudy M, Syka J, Popelar J, et al. Carboplatin and cisplatin ototoxicity in guinea pigs. Audiology. 1992;31(5):293–299. [PubMed] [Google Scholar]
- 39.Delb W, Feilen S, Koch A, et al. Comparative studies of the ototoxicity of cisplatin and carboplatin. Laryngorhinootologie. 1993;72(1):24–27. doi: 10.1055/s-2007-997847. [DOI] [PubMed] [Google Scholar]
- 40.Thomas JP, Lautermann J, Liedert B, et al. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Pharmacol. 2006;70(1):23–29. doi: 10.1124/mol.106.022244. [DOI] [PubMed] [Google Scholar]
- 41.Pochop P, Darsova D, Kukacka J, et al. Intravitreal carboplatin concentration and area under concentration versus time curve after intravitreal and periocular delivery. Eur J Ophthalmol. 2010;20(4):745–751. doi: 10.1177/112067211002000416. [DOI] [PubMed] [Google Scholar]
- 42.Carey JP, Cooper T, Jallo GI, et al. Ototoxicity of carboplatin delivered locally in a monkey brainstem. Int J Toxicol. 2005;24(6):443–449. doi: 10.1080/10915810500368951. [DOI] [PubMed] [Google Scholar]
- 43.Schurig JE, Schlein A, Florczyk AP, et al. Animal models for evaluating the myelosuppressive effects of cancer chemotherapeutic agents. Exp Hematol. 1985;13(Suppl 16):101–105. [PubMed] [Google Scholar]
- 44.Shimoyama Y, Kubota T, Inoue S, et al. Antitumor activity of cisplatin and carboplatin against human tumor xenografts serially transplanted into nude mice--with special reference to gastric carcinomas. Gan To Kagaku Ryoho. 1987;14(9):2682–2687. [PubMed] [Google Scholar]
- 45.Kawano S, Kohmura H, Ohta S, et al. Antigenicity study of carboplatin in guinea pigs and mice. J Toxicol Sci. 1988;13(Suppl 2):1–21. doi: 10.2131/jts.13.supplementii_1. [DOI] [PubMed] [Google Scholar]
- 46.Raynaud FI, Boxall FE, Goddard PM, et al. cis-Amminedichloro(2-methylpyridine) platinum(II) (AMD473), a novel sterically hindered platinum complex: in vivo activity, toxicology, and pharmacokinetics in mice. Clin Cancer Res. 1997;3(11):2063–2074. [PubMed] [Google Scholar]
- 47.Spankuch B, Heim S, Kurunci-Csacsko E, et al. Down-regulation of Polo-like kinase 1 elevates drug sensitivity of breast cancer cells in vitro and in vivo. Cancer Res. 2006;66(11):5836–5846. doi: 10.1158/0008-5472.CAN-06-0343. [DOI] [PubMed] [Google Scholar]
- 48.Vasin MV, Ushakov IB, Kovtun VY, et al. Effect of radioprotector indralin on carboplatinum hemotoxicity. Bull Exp Biol Med. 2006;141(4):437–439. doi: 10.1007/s10517-006-0193-1. [DOI] [PubMed] [Google Scholar]
- 49.Chiu LL, Cunningham LL, Raible DW, et al. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9(2):178–190. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding D, Salvi R. Review of cellular changes in the cochlea due to aminoglycoside antibiotics. The Volta Review. 2005;105(3):407–438. [Google Scholar]
- 51.Ding D, Jiang H, Wang P, et al. Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res. 2007;226(1–2):129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259(1–2):16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding D, Allman A, Yin Sh, et al. Cisplatin ototoxicity. Nova Science Publishers, Inc. 2011, Chapter 2(Hearing Loss Classification, Causes and Treatment) :39–63. [Google Scholar]
- 54.Hatzopoulos S, Petruccelli J, Laurell G, et al. Electrophysiological findings in the Sprague-Dawley rat induced by moderate-dose carboplatin. Hear Res. 2003;182(1–2):48–55. doi: 10.1016/s0378-5955(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 55.Husain K, Whitworth C, Somani SM, et al. Partial protection by lipoic acid against carboplantin-induced ototoxicity in rats. Biomed Environ Sci. 2005;18(3):198–206. [PubMed] [Google Scholar]
- 56.Wake M, Takeno S, Ibrahim D, et al. Carboplatin ototoxicity: an animal model. J Laryngol Otol. 1993;107(7):585–589. doi: 10.1017/s0022215100123771. [DOI] [PubMed] [Google Scholar]
- 57.Takeno S, Harrison RV, Ibrahim D, et al. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75(1–2):93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 58.Takeno S, Harrison RV, Mount RJ, et al. Induction of selective inner hair cell damage by carboplatin. Scanning Microsc. 1994;8(1):97–106. [PubMed] [Google Scholar]
- 59.Wake M, Takeno S, Ibrahim D, et al. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope. 1994;104(4):488–493. doi: 10.1288/00005537-199404000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Ding D, Wang J, Salvi RJ, et al. Early damage in the chinchilla vestibular sensory epithelium from carboplatin. Audiol Neurootol. 1997;2(3):155–167. doi: 10.1159/000259238. [DOI] [PubMed] [Google Scholar]
- 61.Hofstetter P, Ding D, Powers N, et al. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112(1–2):199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 62.Hofstetter P, Ding D, Salvi RJ. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36(6):301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- 63.Hofstetter P, Ding D, Salvi RJ. Induction of spontaneous otoacoustic emissions in chinchillas from carboplatin-induced inner hair cell loss. Hear Res. 2000;150(1–2):132–136. doi: 10.1016/s0378-5955(00)00201-x. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181(1–2):65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Powers NL, Hofstetter P, et al. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107(1–2):67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 66.Ding D, Wang J, Salvi RJ, et al. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 67.Salvi RJ, Wang J, Ding D, et al. Auditory deprivation of the central auditory system resulting from selective inner hair cell loss: animal model of auditory neuropathy. Scand Audiol Suppl. 1999;51:1–12. [PubMed] [Google Scholar]
- 68.Spicer SS, Salvi RJ, Schulte BA. Ablation of inner hair cells by carboplatin alters cells in the medial K(+) flow route and disrupts tectorial membrane. Hear Res. 1999;136(1–2):139–150. doi: 10.1016/s0378-5955(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 69.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147(1–2):261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 70.Spicer SS, Salvi RJ, Schulte BA. Ultrastructural changes in the spiral limbus associated with carboplatin-induced ablation of inner hair cells. Cell Tissue Res. 2000;302(1):1–10. doi: 10.1007/s004410000253. [DOI] [PubMed] [Google Scholar]
- 71.Ding D, Jiang H, Wang J, et al. Carboplatin-induced vestibular damage: Quantitative measurement of type I hair cell loss and ganglion cell loss. Journal of Audiology and Speech Pathology. 2002;10(3):170–173. [Google Scholar]
- 72.Bauer CA, Brozoski TJ. Cochlear structure and function after round window application of ototoxins. Hear Res. 2005;201(1–2):121–131. doi: 10.1016/j.heares.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Ding D, Wu H, et al. Carboplatin-induced injury patterns of inner hair cells missing in chinchilla cochlea. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19(10):457–460. [PubMed] [Google Scholar]
- 74.Ding D, Qi W, Qu Y, et al. Carboplatin and its ototoxicity. Chinese Journal of Otology. 2008;6(2):134–144. [Google Scholar]
- 75.Mount RJ, Takeno S, Wake M, et al. Carboplatin ototoxicity in the chinchilla: lesions of the vestibular sensory epithelium. Acta Otolaryngol Suppl. 1995;519:60–65. doi: 10.3109/00016489509121871. [DOI] [PubMed] [Google Scholar]
- 76.Jiang H, Ding D, Salvi RJ. Carboplatin induced cochlear hair cell lesion in organotypic cultures. Association for Research in Otolaryngology Abstract book. 2006:193. [Google Scholar]
- 77.Jiang H, Ding D, Fu Y, et al. Ototoxic effects of carboplatin in organotypic cultures in rats and chinchillas. Abstr Assoc Res Otolaryngol Abstract book. 2010:245. [Google Scholar]
- 78.Moon IJ, Kim KR, Chu HS, et al. N-acetylcysteine and N-nitroarginine methyl ester attenuate Carboplatin-induced ototoxicity in dissociated spiral ganglion neuron cultures. Clin Exp Otorhinolaryngol. 2011;4(1):11–17. doi: 10.3342/ceo.2011.4.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding D, Qi W, Yu D, et al. Ototoxic effects of mefloquine in cochlear organotypic cultures. Journal of Otology. 2009;4(2):29–38. [Google Scholar]
- 80.Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164(1–2):115–126. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- 81.Ding D, Roth J, Salvi RJ. Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology. 2011;32(2):233–241. doi: 10.1016/j.neuro.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding D, Allman BL, Salvi RJ. Review: Ototoxic Characteristics of Platinum Antitumor Drugs. Anat Rec (Hoboken) 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- 83.Ding D, Fu Y, Jiang H, et al. Oxaliplatin ototoxicity in rat cochlear organotypic cultures. Abstr Assoc Res Otolaryngol Abstract book. 2010:247. [Google Scholar]
- 84.Ding D, He J, Allman BL, et al. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res. 2011;282(1–2):196–203. doi: 10.1016/j.heares.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding D, Qi W, Zhang M, et al. Cisplatin and its ototoxicity. Chinese Journal of Otology. 2008;6(2):125–133. [Google Scholar]
- 86.McFadden SL, Kasper C, Ostrowski J, et al. Effects of inner hair cell loss on inferior colliculus evoked potential thresholds, amplitudes and forward masking functions in chinchillas. Hear Res. 1998;120(1–2):121–132. doi: 10.1016/s0378-5955(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 87.Ding L, McFadden SL, Salvi RJ. Calpain immunoreactivity and morphological damage in chinchilla inner ears after carboplatin. J Assoc Res Otolaryngol. 2002;3(1):68–79. doi: 10.1007/s101620020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y, Godfrey DA, Godfrey MA, et al. Effects of carboplatin on amino acid chemistry in chinchilla cochlear nucleus. Hear Res. 2002;165(1–2):19–29. doi: 10.1016/s0378-5955(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 89.Saito T, Manabe Y, Honda N, et al. Semiquantitative analysis by scanning electron microscopy of cochlear hair cell damage by ototoxic drugs. Scanning Microsc. 1995;9(1):271–280. discussion 280-271. [PubMed] [Google Scholar]
- 90.Ishida S, Lee J, Thiele DJ, et al. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99(22):14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin X, Okuda T, Holzer A, et al. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol. 2002;62(5):1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 92.Petris MJ, Voskoboinik I, Cater M, et al. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem. 2002;277(48):46736–46742. doi: 10.1074/jbc.M208864200. [DOI] [PubMed] [Google Scholar]
- 93.Guo Y, Smith K, Petris MJ. Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem. 2004;279(45):46393–46399. doi: 10.1074/jbc.M407777200. [DOI] [PubMed] [Google Scholar]
- 94.Holzer AK, Samimi G, Katano K, et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2004;66(4):817–823. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- 95.Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006;234(1):34–39. doi: 10.1016/j.canlet.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 96.Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol He-matol. 2005;53(1):13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Safaei R, Otani S, Larson BJ, et al. Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol. 2008;73(2):461–468. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- 98.Kuo MT, Chen HH, Song IS, et al. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26(1):71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 99.Owatari S, Akune S, Komastsu M, et al. Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res. 2007;67(10):4860–4868. doi: 10.1158/0008-5472.CAN-06-3096. [DOI] [PubMed] [Google Scholar]
- 100.Yoshizawa K, Nozaki S, Kitahara H, et al. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol Rep. 2007;18(4):987–991. [PubMed] [Google Scholar]
- 101.Zisowsky J, Koegel S, Layers S, et al. Relevance of drug uptake and efflux for cisplatin sensitivity of tumor cells. Biochem Pharmacol. 2007;73(2):298–307. doi: 10.1016/j.bcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 102.He J, Ding D, Yu D, et al. Modulation of copper transporters in protection against cisplatin-induced cochlear hair cell damage. Journal of Otology. 2011;6(2):53–61. [Google Scholar]
- 103.Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6(2):171–180. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 104.Ding D, Jiang H, MeFadden SL, et al. Ethacrynic acid is the key for opening of the blood-labyrinth barrier. Chinese Journal of Otology. 2004;1(2):42–47. [Google Scholar]
- 105.Ding D, Jiang H, Salvi RJ. Local application of copper sulfate protects cochlear hair cells against carboplatin in chinchillas. Abstr Assoc Res Otolaryngol Abstract book. 2012:56. [Google Scholar]
- 106.Ding D, McFadden SL, Browne RW, et al. Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hear Res. 2003;185(1–2):90–96. doi: 10.1016/s0378-5955(03)00258-2. [DOI] [PubMed] [Google Scholar]
- 107.Ding D, McFadden SL, Woo JM, et al. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear Res. 2002;173(1–2):1–9. doi: 10.1016/s0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 108.Ding D, Wu X, Liu H, et al. Ototoxicity and neurotoxicity of nedaplatin in cochlear organotypic cultures. Abstr Assoc Res Otolaryngol Abstract book. 2011:208. [Google Scholar]
- 109.Clerici WJ, Hensley K, DiMartino DL, et al. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98(1–2):116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 110.Kopke RD, Liu W, Gabaizadeh R, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18(5):559–571. [PubMed] [Google Scholar]
- 111.Dehne N, Lautermann J, Petrat F, et al. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide an-ion radicals. Toxicol Appl Pharmacol. 2001;174(1):27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- 112.Li G, Liu W, Frenz D, et al. Cisplatin ototoxicity to the rat inner ear: a role for HMG1 and iNOS. Neurotoxicology. 2006;27(1):22–30. doi: 10.1016/j.neuro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Jamesdaniel S, Coling D, Hinduja S, et al. Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J Biol Chem. 2012;287(22):18674–18686. doi: 10.1074/jbc.M111.297960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995;76(6):386–394. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 115.Campbell KC, Rybak RP, Rybak LP, et al. D-methionine protects against cisplatin damage to the stria vascularis. Hear Res. 1999;138(1–2):13–28. doi: 10.1016/s0378-5955(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 116.Kemp G, Rose P, Lurain J, et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol. 1996;14(7):2101–2112. doi: 10.1200/JCO.1996.14.7.2101. [DOI] [PubMed] [Google Scholar]
- 117.Rybak LP, Husain K, Whitworth C, et al. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sci. 1999;47(2):195–202. doi: 10.1093/toxsci/47.2.195. [DOI] [PubMed] [Google Scholar]
- 118.Li G, Frenz DA, Brahmblatt S, et al. Round window membrane delivery of L-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotoxicology. 2001;22(2):163–176. doi: 10.1016/s0161-813x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 119.Aamdal S, Fodstad O, Pihl A, et al. Some procedures to reduce cis-platinum toxicity reduce antitumour activity. Cancer Treat Rev. 1987;14(3–4):389–395. doi: 10.1016/0305-7372(87)90035-1. [DOI] [PubMed] [Google Scholar]
- 120.Basinger MA, Jones MM, Holscher MA, et al. L-methionine antagonism of cis-platinum nephrotoxicity. Toxicol Appl Pharmacol. 1990;103(1):1–15. doi: 10.1016/0041-008x(90)90257-u. [DOI] [PubMed] [Google Scholar]
- 121.Liu W, Staecker H, Stupak H, et al. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport. 1998;9(11):2609–2614. doi: 10.1097/00001756-199808030-00034. [DOI] [PubMed] [Google Scholar]
- 122.Lau AH. Apoptosis induced by cisplatin nephrotoxic injury. Kidney Int. 1999;56(4):1295–1298. doi: 10.1046/j.1523-1755.1999.00687.x. [DOI] [PubMed] [Google Scholar]
- 123.Alam SA, Ikeda K, Oshima T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141(1–2):28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez VM, Fuertes MA, Alonso C, et al. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59(4):657–663. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- 125.Park SA, Park HJ, Lee BI, et al. Bcl-2 blocks cisplatin-induced apoptosis by suppression of ERK-mediated p53 accumulation in B104 cells. Brain Res Mol Brain Res. 2001;93(1):18–26. doi: 10.1016/s0169-328x(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 126.Hershberger PA, McGuire TF, Yu WD, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1(10):821–829. [PubMed] [Google Scholar]
- 127.Lee JE, Nakagawa T, Kim TS, et al. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113(6):994–999. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 128.Xu HJ, Huang WN. Cisplatin-induced apoptotic cell death in spiral ganglion and organ of Corti of mongolian gerbil cochlear. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2003;38(2):98–100. [PubMed] [Google Scholar]
- 129.Zhang M, Liu W, Ding D, et al. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120(1):191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- 130.Arany I, Megyesi JK, Kaneto H, et al. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2004;287(3):F543–F549. doi: 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- 131.Marklund L, Andersson B, Behnam-Motlagh P, et al. Cellular potassium ion deprivation enhances apoptosis induced by cisplatin. Basic Clin Pharmacol Toxicol. 2004;94(5):245–251. doi: 10.1111/j.1742-7843.2004.pto940508.x. [DOI] [PubMed] [Google Scholar]
- 132.Kim YK, Kim HJ, Kwon CH, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol. 2005;25(5):374–382. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
- 133.Yu F, Megyesi J, Safirstein RL, et al. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2005;289(3):F514–F520. doi: 10.1152/ajprenal.00101.2005. [DOI] [PubMed] [Google Scholar]
- 134.Jiang M, Wei Q, Wang J, et al. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25(29):4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- 135.Jirsova K, Mandys V, Gispen WH, et al. Cisplatin-induced apoptosis in cultures of human Schwann cells. Neurosci Lett. 2006;392(1–2):22–26. doi: 10.1016/j.neulet.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 136.Ding D, Wang P, Jiang H, et al. Gene expression in cisplatin ototoxicity and protection with p53 inhibitor. Chinese Journal of Otology. 2009;4(2):15–24. [Google Scholar]
- 137.Xu L, Kong D, Zhu L, et al. Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem. 2007;295(1–2):153–165. doi: 10.1007/s11010-006-9285-5. [DOI] [PubMed] [Google Scholar]
- 138.Yang C, Kaushal V, Shah SV, et al. Mcl-1 is downregulated in cisplatin-induced apoptosis, and proteasome inhibitors restore Mcl-1 and promote survival in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292(6):F1710–F1717. doi: 10.1152/ajprenal.00505.2006. [DOI] [PubMed] [Google Scholar]
- 139.Yano T, Itoh Y, Matsuo M, et al. Involvement of both tumor necrosis factor-alpha-induced necrosis and p53-mediated caspase-dependent apoptosis in nephrotoxicity of cisplatin. Apoptosis. 2007;12(10):1901–1909. doi: 10.1007/s10495-007-0110-8. [DOI] [PubMed] [Google Scholar]
- 140.Gao WQ, Dybdal N, Shinsky N, et al. Neurotrophin-3 reverses experimental cisplatin-induced peripheral sensory neuropathy. Ann Neurol. 1995;38(1):30–37. doi: 10.1002/ana.410380108. [DOI] [PubMed] [Google Scholar]
- 141.Zheng JL, Gao WQ. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur J Neurosci. 1996;8(9):1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 142.Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplatin neurotoxicity. J Neurosci. 1995;15(7 Pt 2):5079–5087. doi: 10.1523/JNEUROSCI.15-07-05079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]