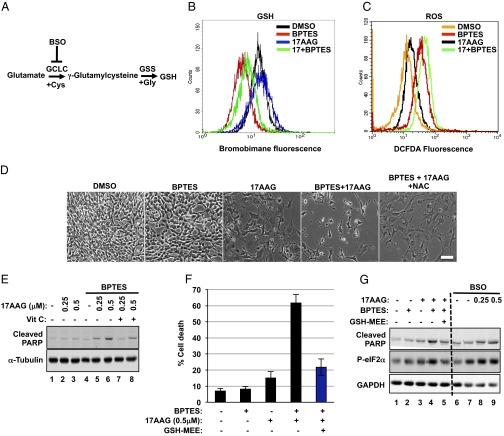
Fig. 5.
Deregulated redox balance is responsible for the apoptosis induced by BPTES and 17AAG. (A) A diagram showing the enzymes involved in the biosynthesis of GSH and the inhibitor used in this study (see text for more details). (B) Intracellular GSH levels were measured in Tsc2−/− MEFs treated with DMSO, BPTES (10 μM), 17AAG (0.5 μM), or BPTES plus 17AAG for 48 h (n = 3). (C) Intracellular ROS levels were measured in Tsc2−/− MEFs treated as in B (n = 3). (D) Tsc2−/− MEFs were treated with DMSO, 17AAG (0.5 μM), BPTES (10 μM), and NAC (10 mM) as indicated for 72 h. Phase microscopy was used to observe cell viability. (E) Immunoblot analysis of cleaved PARP and α-tubulin in Tsc2−/− MEFs treated for 24 h with DMSO, 17AAG (0.5 μM), BPTES (10 μM), and vitamin C (100 μM) as indicated. (F) Cell death of Tsc2−/− MEFs treated with DMSO, 17AAG (0.5 μM), BPTES (10 μM), and GSH-MEE (2 mM) for 72 h. The mean is shown; error bars represent SEM (n = 3). (G) Immunoblot analysis of cleaved PARP, p-eIF2α, and GAPDH in Tsc2−/− MEFs treated for 24 h as in F (lanes 1–5) or with BSO (1 mM) combined with increasing concentrations of 17AAG (lanes 6–9).