Table 1. Enantioselective C–H Insertion with Diazo Ester 9a.
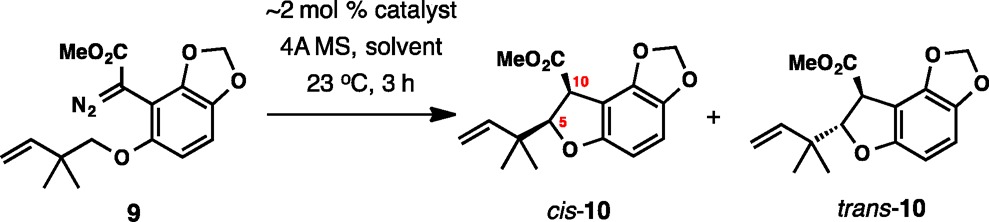
| entry | catalyst | solvent | yield (%)a | cis:trans | ee, cis (%) | ee, trans (%) |
|---|---|---|---|---|---|---|
| 1 | Rh2(S-PTTL)4 | CH2Cl2 | 25 | 9.1:1 | 70 | 50 |
| 2 | Rh2(S-PTTL)4 | EtOAc | trace | |||
| 3 | Rh2(S-PTTL)4 | hexane | 34 | 6.7:1 | 51 | |
| 4 | Rh2(R-PTTL)4 | PhMe | 47 | 5.9:1 | −60 | −33 |
| 5 | Rh2(S-NTTL)4 | CH2Cl2 | 27 | 9.1:1 | 54 | |
| 6 | Rh2(S-TCPTTL)4 | CH2Cl2 | 42 | 30 | 18 | |
| 7 | Rh2(R-BPTV)4 | CH2Cl2 | trace | |||
| 8 | Rh2(S-PTTL)4 | CH2Cl2c | 53 | 10.0:1 | 60 | |
| 9 | Rh2(S-PTAD)4 | CH2Cl2c | 22 | 3.7:1 | 48 | |
| 10 | Rh2(R-BTPCP)4 | CH2Cl2c | NR | |||
| 11 | Rh2(R-BPTV)4 | CH2Cl2c | trace | |||
| 12 | Rh2(S-biTISP)2 | CH2Cl2c | trace | 1:1 | ||
| 13 | Rh2(S-TCPTAD)4 | CH2Cl2c | 24 | 3:1 | 8 |
Diastereomer ratios and enantiomeric excess were determined by HPLC analysis.
Combined isolated yield of cis-10 and trans-10.
At reflux.
