Table 2. Enantioselective C–H Insertion with Diazo Esters 11a.
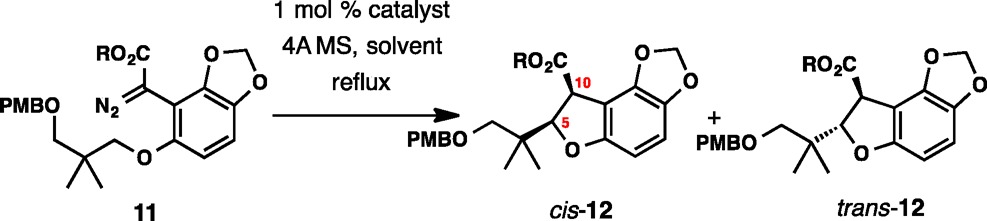
| entry | R | catalyst | solvent | yield %b | cis:trans | ee, cis (%) | ee, trans (%) |
|---|---|---|---|---|---|---|---|
| 1 | Me | Rh2(S-PTTL)4 | DMB | 71 | 1.4:1 | 45 | 80 |
| 2 | Me | Rh2(S-PTTL)4 | CHCl3 | 63 | 1.6:1 | 49 | 67 |
| 3 | Me | Rh2(S-PTTL)4 | c-C6H12 | 69 | 1.6:1 | 41 | 77 |
| 4 | t-Bu | Rh2(S-PTTL)4 | DMB | 55 | 3.0:1 | <5 | 66 |
| 5 | t-Bu | Rh2(S-PTTL)4 | CHCl3 | 28 | 3.0:1 | <5 | 57 |
| 6 | t-Bu | Rh2(S-PTTL)4 | c-C6H12 | 70 | 3.5:1 | 23 | 59 |
| 7 | Me | Rh2(S-PTA)4 | c-C6H12 | 79 | 1.0:1 | 18 | 57 |
| 8 | Me | Rh2(R-PTV)4 | c-C6H12 | 76 | 1:1.1 | ∼42 | ∼78 |
| 9 | Me | Rh2(S-NTTL)4 | c-C6H12 | 74 | 2.2:1 | 29 | 56 |
| 10 | Me | Rh2(S-PTAD)4 | c-C6H12 | 79 | 1.4:1 | 41 | 54 |
| 11 | Me | Rh2(R-BPTV)4 | c-C6H12 | 76 | 1:1.1 | ∼43 | ∼80 |
| 12 | Me | Rh2(S-biTISP)4 | c-C6H12 | 78 | 1:1.4 | ∼10 | 38 |
| 13 | Me | Rh2(S-BTPCP)4 | c-C6H12 | 65 | 3.8:1 | ∼36 | ∼11 |
| 14 | Me | Rh2(R-DPCP)4 | c-C6H12 | 74 | 1:1.1 | 19 | ∼18 |
| 15 | Me | Rh2(S-DOSP)4 | c-C6H12 | 71 | 1.0:1 | <5 | 7 |
| 16 | Me | Rh2(S-BNP)4 | c-C6H12 | 73 | 1.9:1 | ∼19 | ∼45 |
| 17 | t-Bu | Rh2(S-BTPCP)4 | c-C6H12 | 77 | 2.8:1 | 42 | 63 |
| 18 | t-Bu | Rh2(S-biTISP)2 | c-C6H12 | 77 | 2.5:1 | ∼46 | 46 |
| 19 | t-Bu | Rh2(S-BNP)4 | c-C6H12 | 83 | 3.3:1 | 13 | 24 |
Diastereomer ratios determined by 1H NMR analysis of crude reaction mixture. Enantiomeric excesses were determined by HPLC analysis.
Combined isolated yield of cis-12 and trans-12.
