Table 3. Lactamide and Mandelamide as Chiral Auxiliaries .
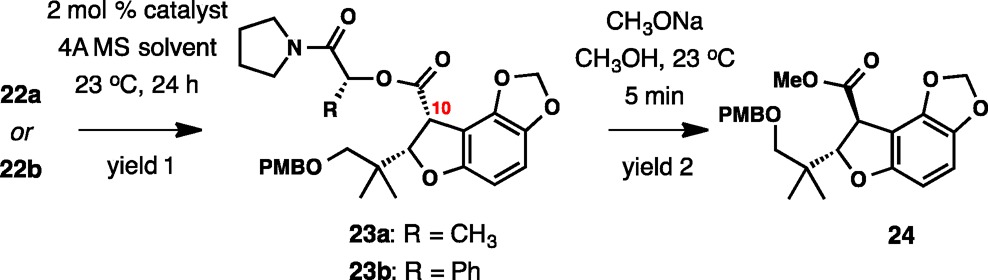
| entry | starting material | catalyst | solvent | yield 1 (%)a | yield 2 (%)b | ee %e |
|---|---|---|---|---|---|---|
| 1 | 22a | Rh2(S-PTAD)4 | CH2Cl2 | <10 | ||
| 2 | 22a | Rh2(R-PTAD)4 | CH2Cl2 | <10 | ||
| 3 | 22a | Rh2(S-DOSP)4 | CH2Cl2 | 63 | 83 | 62 |
| 4 | 22a | Rh2(R-DOSP)4 | CH2Cl2 | 74 | 86 | 70 |
| 5 | 22a | Rh2(OAc)4 | CH2Cl2 | 84 | 84 | 65 |
| 6 | 22a | Rh2(O2CC4F9)4 | CH2Cl2 | 0 | ||
| 7 | 22a | Rh2(R-DOSP)4 | (CH2Cl)2 | 72 | ||
| 8 | 22a | Rh2(R-DOSP)4 | PhMe | 61 | ||
| 9 | 22a | Rh2(R-DOSP)4 | c-C6H12 | <10 | ||
| 10 | 22a | Rh2(R-DOSP)4 | CH3CN | 0 | ||
| 11 | 23b | Rh2(S-DOSP)4 | CH2Cl2 | 84 | 89 | 59 |
| 12 | 23b | Rh2(R-DOSP)4 | CH2Cl2 | 57 | 77 | 52 |
| 13 | 23b | Rh2(OAc)4 | CH2Cl2 | 72 | 85c | 76 |
| 14 | 23b | Rh2(OAc)4 | CH2Cl2 | 61d | 84 | 84 |
Isolated yield.
Combined isolated yield.
Isolated yield, complete isomerization to the trans isomer observed.
Major diastereomer isolated in pure form by column chromatography.
Determined by HPLC.
