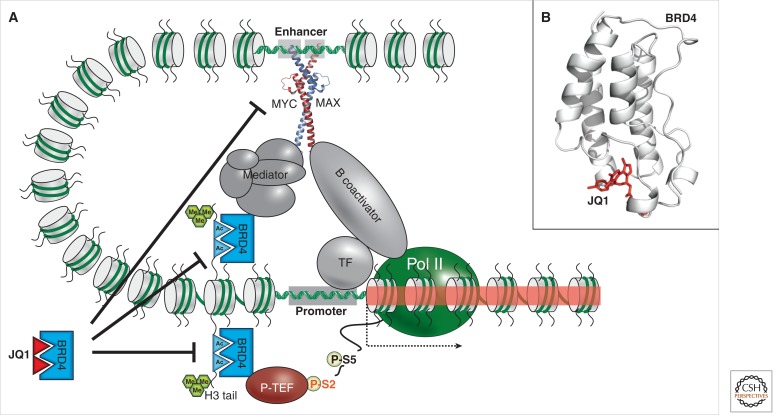
Figure 1.
Model for JQ1 small-molecule inhibition of the BRD4 bromodomain in cancer. (A) The aberrant transcriptional activation of MYC target genes (indicated as red shaded regions of chromatin) is a common feature of many cancers. Transcriptional activation requires the bromodomain “reading” function of BRD4, which recognizes acetylation marks (“Ac”-labeled cyan triangles) on histone H3 tails at promoter-proximal target sequences. Acetyl-bound BRD4 interacts with both the MYC-MAX complex bound to enhancer sequences (via a mediator complex) and the PTEFb phosphorylase required for the release of RNA polymerase II (Pol II) during transcriptional elongation. The competitive binding of the JQ1 bromodomain inhibitor (red triangle) to BRD4 not only reduces transcription of the MYC gene (top inhibition arrow) but also its target genes by abrogating recruitment of enhancer complexes and PTEFb (middle and bottom inhibition arrows), possibly via chromatin looping. The active chromatin mark histone H3K4me3 is illustrated as triple green hexagons. (B) Crystal structure of the human BRD4 protein in complex with JQ1 (red).