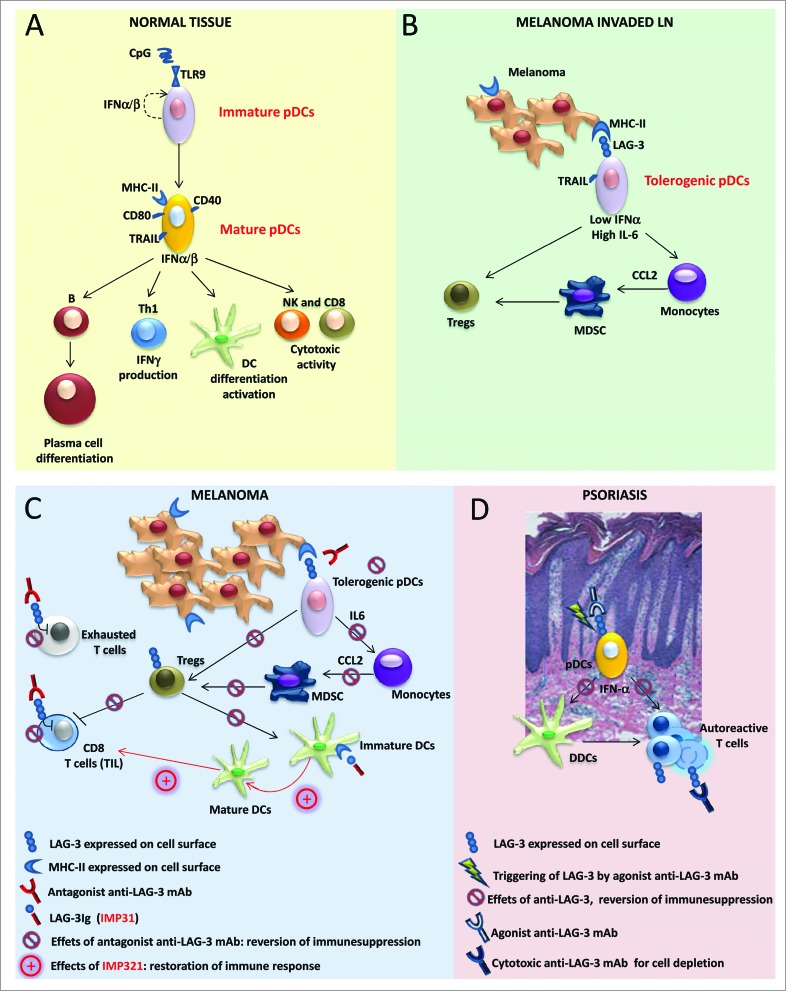
Figure 1 (See previous page).
Role of LAG-3 in pDCs and its translational relevance in melanoma and psoriasis. (A) Under normal conditions, upon activation, plasmacytoid dendritic cells (pDCs) upregulate T cell co-stimulatory molecules (such as CD86 and CD40) and increase MHC-II and TRAIL expression; activated pDCs secrete pro-inflammatory and anti-viral cytokines, such as Type I interferons (IFNα/β), and promote B- and DC-cell differentiation/activation, T helper cell type 1 (Th1) response, and natural killer (NK) cell and CD8+ T cell cytotoxic activities. (B) In melanoma-invaded lymph nodes (LNs), MHC-II molecules on melanoma cells bind to lymphocyte activated gene 3 (LAG-3) expressed on the surface of pDCs, resulting in the tolerogenic activation of pDCs. Low levels of IFNα and high levels of interleukin 6 (IL-6) induce monocytes to produce chemokine (C-C) motif ligand 2 (CCL2) with subsequent myeloid derived suppressor cell (MDSC) accumulation and regulatory T cell (Treg) expansion. (C) In melanoma lesions, the antagonistic anti-LAG-3 mAb blocks the negative signals mediated by LAG-3 in exhausted T cells and limits the suppressor functions of Tregs. The same mAb blocks the interaction between pDCs expressing LAG-3 and melanoma cells expressing its ligand (MHC-II), allowing for pDC activation and IFNα production while limiting the frequencies of MDSCs and Tregs with subsequent T-cell expansion. Clinical-grade LAG-3Ig (IMP321) promotes DC maturation and CD8+ T-cell induction/expansion. (D) In psoriatic lesions, the agonist anti-LAG-3 mAb can trigger LAG-3-mediated signaling in pDCs, thus diminishing IFNα production and consequently hampering the activation of dermal dendritic cells (DDCs) and the development of pathogenic Th1 responses. The cytotoxic anti-LAG-3 mAb depletes autoreactive LAG-3+ T cells.