Abstract
Androgens negatively affect both central and peripheral immunity. Their potent immunosuppressive ability is thought to lead to gender dimorphism in autoimmune and infectious disease and hamper immunosurveillance of tumors. Therefore, we investigated the molecular underpinnings of androgens suppression of T-cell differentiation and found evidence that developing strategies to counteract these inhibitory effects may foster successful cancer immunotherapy in the future.
Keywords: androgens, immunosuppression, lymphocyte differentiation, PTPN1 phosphatase
Immune modulation by androgens has been explored previously in both animal models and humans. Such studies have established androgens as the main drivers of gender dimorphism in autoimmune and infectious diseases, with females generally being more prone to autoimmunity and yet relatively less susceptible to infection than are males.1 In a recent clinical trial, men's antibody response to a flu vaccine was significantly weaker than that observed in women. Interestingly, men with low levels of testosterone had an antibody response that was similar in strength to women's,2 suggesting sex hormone levels could hamper vaccine efficacy. Androgens exhibit immunosuppressive capabilities through their action on both lymphoid and myeloid immune cells in the periphery. Additionally, androgens also induce thymic involution by causing a decline in the input of bone-marrow-derived stem cells into the gland and a loss of thymic epithelial cells, thus altering cell trafficking, reducing thymocyte proliferation, and increasing thymocyte apoptosis.3 More recently it has become increasingly evident that androgen deprivation results in re-expansion of the involuted thymus of adult animals and men, leading to measurable changes in the pool of circulating T cells. In the periphery, lymphocytes undergo testosterone- and dihydrotestosterone- (DHT) mediated suppression by the virtue of their expression of both the cytosolic and membrane forms of the androgen receptor (AR). For example, testosterone treatment of patients with multiple sclerosis significantly reduced IL-2 and increased transforming growth factor β1 (TGFβ1) production.4 It was later shown in multiple mouse models that androgen deprivation ameliorated immunosurveillance of prostate tumors by circumventing immune tolerance to tumor antigens in immunized animals.5-7 Consequently, the idea of combining hormone therapy with immunotherapy and other standard cancer therapies is now being clinically tested in prostate cancer patients.
To date, very little is known about the cell signaling events that mediate androgen's immunogenic effects. Recent reports have shown that gender dichotomy in CD4+ T-cell differentiation was regulated through peroxisome proliferator–activated receptor α (PPARα). PPARα is more abundant in male-derived CD4+ T cells as compared to those originating in females and its expression is sensitive to androgen levels. Deletion of PPARα selectively removes the brake on nuclear factor κB (NF-κB) and c-Jun activity in male T lymphocytes, resulting in higher production of interferon γ (IFNγ) and tumor necrosis factor α (TNFα).8 It was later shown that human T cells exhibit a sex difference in the production of IFNγ and IL-17A that may be driven by differential expression of both PPARα and PPARγ.9
We have recently identified a new signaling mechanism that is triggered by testosterone in T cells.10 In an attempt to identify potential regulators of androgen-mediated immunosuppression, we profiled the transcriptome of CD4+ T cells from surgically castrated and control (intact) male mice and performed pathway analysis. We found that androgen deprivation dramatically altered gene expression patterns that regulate T-cell differentiation. We further analyzed the effects of androgen on murine and human T cell differentiation in vitro using testosterone-free culture conditions and testosterone supplementation and found that testosterone suppresses T helper type 1 (Th1) differentiation, resulting in reduced expression of Th1 markers T-bet, IFNγ, IL-12Rβ and IL-18R1.10 In addition, testosterone reduced the phosphorylation rate of tyrosine kinase 2 (TYK2) and signal transduction and activator of transcription 4 (STAT4) in response to IL-12 challenge, and reduced the threshold of IL-12 required to induce Th1 differentiation. The latter finding might explain our observation that T cells infiltrate various tissues in castrated mice, even in the absence of antigen priming. Many differentially expressed genes that contribute to regulation of Th1 differentiation are related to the JAK/STAT pathway. Among these, , protein-tyrosine phosphatase non-receptor type 1 (PTPN1, also known asPTP1B), was downregulated in CD4+ T cells from both castrated mice and prostate cancer patients undergoing androgen deprivation therapy (ADT). Consistently, testosterone up-regulated PTPN1 expression in vitro. PTPN1 can dephosphorylate the phosphotyrosine residues of many kinases including insulin receptor kinase, leptin receptor, epidermal growth factor receptor, insulin-like growth factor 1 receptor, colony stimulating factor 1 receptor, c-Src, and focal adhesion kinase as well as other tyrosine-phosphorylated proteins, including STAT5, BCAR1, DOK1, β-catenin and cortactin. Interestingly, PTPN1 also dephosphorylates JAK2 and TYK2, 2 JAK/STAT pathway kinases that play a major role in lymphocyte responses to IL-12 and IL-23 and Th1 and Th17 cell-fate specification. More importantly, we showed that pharmacological inhibition of PTPN1 in the presence of testosterone restoresIL-12-induced TYK2 and STAT4 phosphorylation, thus establishing PTPN1 as a direct mediator of testosterone-mediated suppression of Th1 differentiation.10 Finally, we identified an androgen receptor-binding site in the intron between exons 3 and 4 of the Ptpn1 gene, a site that is conserved across multiple species but different from the previously reported sites found in other cell types.
The use of PTPN1 inhibitors for the treatment of type 2 diabetes and obesity in preclinical trials has generated much enthusiasm in the last decade. Yet, this approach has met with a multitude of technical challenges. However, as new, more specific and efficient inhibitors are being generated, it is tempting to speculate that PTPN1 may represent a viable target for cancer immunotherapy, like the other, more popular immune checkpoints cytotoxic T lymphocyte associated protein 4 (CTLA-4), programmed cell death 1 (PD-1) and lymphocyte activation gene 3 (LAG-3). Overall, our findings bring new insight into the mechanisms linking androgens to autoimmunity (Fig. 1), and provide rationale for the combination of PTPN1 inhibitors in conjunction with cancer immunotherapy.
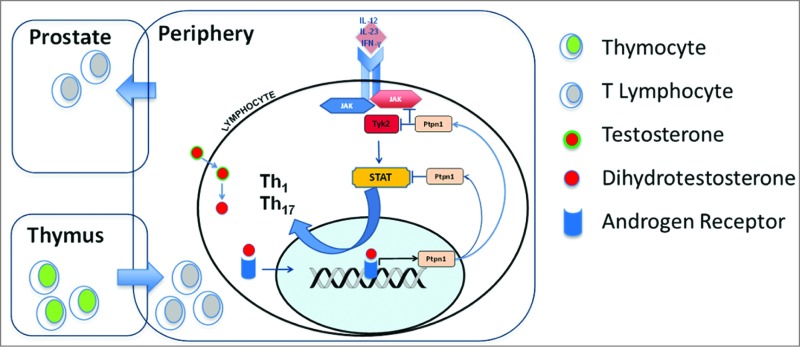
Figure 1.
Multicompartmental action of androgens on the immune system. Androgen-induced immune regulation takes place in the thymus and peripheral immune system by suppressing T helper type 1 differentiation. The expression of the key regulatory molecule protein-tyrosine phosphatase non-receptor type 1 (PTPN1) in T lymphocytes is transcriptionally upregulated by dihydrotestosterone activation of androgen receptor. PTPN1-mediated regulation of Th1 and Th17 cell-fate specification occurs, in turn, via PTPN1 phosphatase activity that attenuates the cytokine-induced JAK/STAT signaling axis, thus dampening Th1 and Th17 differentiation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737; PMID:; http://dx.doi.org/ 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 2013; 111:869; PMID:; http://dx.doi.org/ 10.1073/pnas.1321060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen NJ, Viselli SM, Fan J, Kovacs WJ. Androgens accelerate thymocyte apoptosis. Endocrinology 1998; 139:748; PMID: [DOI] [PubMed] [Google Scholar]
- 4. Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation 2008; 5:32; PMID:; http://dx.doi.org/ 10.1186/1742-2094-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, et al. . Androgen ablation mitigates tolerance to a prostate cancer-restricted antigen. Cancer Cell 2005; 7:239; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2005.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arredouani MS, Tseng-Rogenski SS, Hollenbeck BK, Escara-Wilke J, Leander KR, Defeo-Jones D, Hwang C, Sanda MG. Androgen ablation augments human HLA2.1-restricted T cell responses to PSA self-antigen in transgenic mice. Prostate 2010; 70:1002; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009; 69:571; PMID:; http://dx.doi.org/ 10.1002/pros.20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med 2007; 204:321; PMID:; http://dx.doi.org/ 10.1084/jem.20061839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, et al. . Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A 2012; 109:9505; PMID:; http://dx.doi.org/ 10.1073/pnas.1118458109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A 2014; 111:9887; PMID:; http://dx.doi.org/ 10.1073/pnas.1402468111 [DOI] [PMC free article] [PubMed] [Google Scholar]