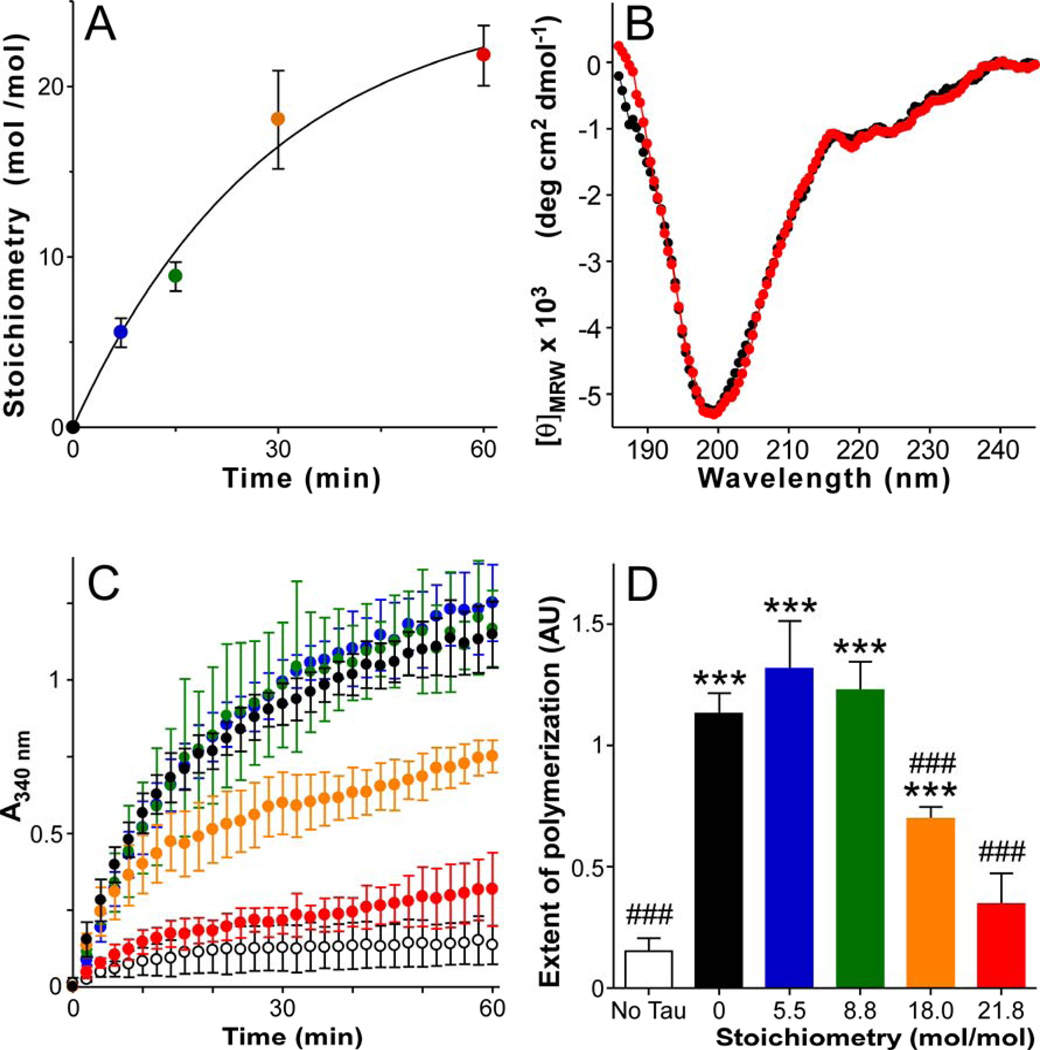
Figure 3. Effect of methylation on tau structure and function in vitro.
Purified recombinant human 2N4R tau protein was subjected to reductive methylation in the presence of identical concentrations of [14C]-labeled or unlabeled formaldehyde at room temperature, then assayed for methylation stoichiometry, secondary structure, and tubulin assembly promoting activity. (A) Time course of methylation determined in the presence of [14C]formaldehyde (n = 3), where the solid line represents best fit of the data with Eq. 1. [14C]methyl incorporation ranged from 0 – 21.8 mol/mol over the 60 min time course. (B) CD spectra (20°C) of methylated and non-methylated tau suspended in 100 mM sodium perchlorate, 20 mM boric acid, pH 7.4. Symbol colors correspond to stoichiometries estimated in panel A. (C) Effect of methylated tau (prepared in unlabeled formaldeyde) on tubulin assembly (37°C) as measured by absorbance (A340 nm). Each data point represents triplicate determination of A340 ± SD as a function of time. Assays were performed in the absence (white circles) or presence of tau containing 0 – 21.8 mol/mol methylation (symbol colors correspond to stoichiometries estimated in panel A). (D) Quantification of tubulin assembly at 60 min (data from Panel C). The extent of tau-mediated tubulin assembly was depressed only at high methylation stoichiometries (≥18 mol/mol). ***, p < 0.001 for comparison with no tau control (hollow bar); ###, p < 0.001 for comparison with non-methylated tau control (black bar).