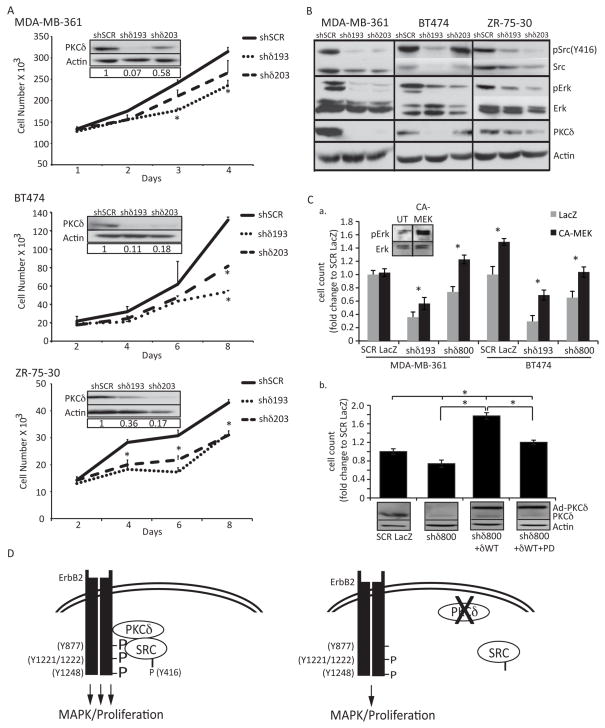
Figure 5. PKCδ regulates proliferation in ErbB2 overexpressing human breast cancer cells.
A. MDA-MB-361, BT-474, and ZR-75-30 cells were depleted of PKCδ as described in Materials and Methods. Cells were plated at equal densities and counted at four time points. Representative experiments shown; experiments were repeated a minimum of three times, p=<0.05. Insets show depletion of PKCδ by immunoblot with actin as a loading control. B. Whole cell lysates from MDA-MB-361, BT-474, and ZR-75-30 cells were resolved on an SDS-page gel and probed with the following antibodies: pSrc (Y416); Src; pERK1/2 (T202/Y204); ERK1/2; PKCδ. Immunoblots probed for phosphorylated proteins were stripped and probed with antibodies to total protein, as indicated. Blots were probed for actin as a loading control. Representative experiments are shown; experiments were repeated a minimum of three times. C., panel a, MDA-MB-361 and BT474 cells with PKCδ knockdown (shδ193 or shδ800) were transduced with an adenovirus encoding either LacZ or a constitutively activated MEK (CA-MEK) MOI=100). Cells were counted on day 5 (BT-474) or day 6 (MDA-MB-361). Inset shows pERK activation upon 48 hours of CA-MEK expression in BT-474 cells. Panel b, MDA-MB-361 cells (SCR and shδ800) were transduced with adenovirus encoding either LacZ or GFP-PKCδWT (MOI=100). On the subsequent day media was replaced and 10uM PD98059 was added to the indicated wells; cells were counted on day 6. Lysates were probed for PKCδ and actin. D. Model: Loss of PKCδ decreases the phosphorylation and binding of Src to the ErbB2 receptor, resulting in decreased ERK signaling and abrogated ErbB2 driven proliferation. See text for detailed discussion.