Figure 3. Possible mechanisms underlying the regulation of AMPAR assembly.
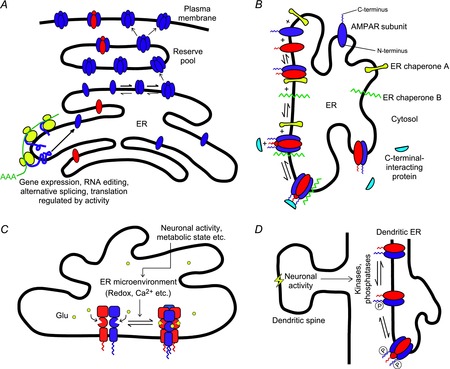
A, the level of gene expression, alternative splicing and/or translation of a particular type of AMPAR subunit (blue) is modulated by an activity-dependent signal, thereby shifting the subunit composition of the reserve receptor pool. B, two hypothetical ER chaperones ‘A’ and ‘B’ bind to and stabilize dimers and tetramers of a particular subunit composition (red and blue), respectively, thereby modulating the equilibrium of tetramerization. C-terminal-interacting proteins might also take part in this process. C, the equilibrium between receptor dimers and tetramers may be influenced by the microenvironment in the ER lumen (e.g. redox, Ca2+, glutamate concentration, etc.), which could in turn be altered by changes in neuronal activity and metabolic state. D, since the C-termini of AMPAR subunits in the ER are exposed to the cytoplasm, their phosphorylation states could be modified by cytoplasmic kinases and phosphatases, whose activities could be influenced by neuronal signals from nearby dendritic spines. The phosphorylation level of the CTD could in turn modulate preferential assembly of different subunits, leading to changes in subunit composition.
