Figure 3. Early structural model of iGluR activation and desensitization.
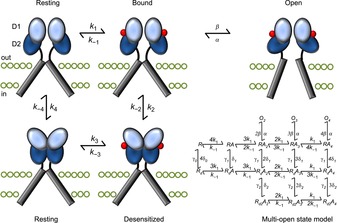
The binding of agonist molecules (red) to the LBD dimer permits subsequent rearrangement to one of two conformations: activated or desensitized. The activated state occurs when the two D2 lobes (dark blue) are lifted apart, generating forces on the transmembrane domains (grey bars) to open the channel pore. Alternatively, the desensitized state stems from the separation of the two D1 domains (light blue) at the dimer interface, relaxing the LBD such that the energy provided by ligand binding cannot open the pore. The structural states are arranged according to the cyclic gating model described in Fig. 1, although to better account for the complex functional behaviour of iGluRs, more advanced kinetic models (Robert & Howe, 2003) have been developed, into which the four agonist binding sites are incorporated (bottom right). Moreover, is not clear to what extent movements of LBD dimers occur in the absence of bound ligands. The ATD has also been excluded for simplicity.
