Abstract
Background
Previous studies have demonstrated the presence of a social cognition factor as an element of general cognition in healthy control and clinical populations. Recently developed measures of social cognition include the Social Perception and Faces subtests of the Wechsler Advanced Clinical Solutions (ACS) Social Cognition module. While these measures have been validated on various clinical samples, they have not been studied in alcoholics. Alcoholism has been associated with emotional abnormalities and diminished social cognitive functioning as well as neuropathology of brain areas underlying social processing abilities. We used the ACS Social Perception and Faces subtests to assess alcoholism-related impairments in social cognition.
Methods
Social cognitive functioning was assessed in 77 abstinent alcoholic individuals (37 women) and 59 nonalcoholic control participants (29 women), using measures of the ACS Social Cognition module and subtests of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) that contain a social cognition component (Picture Completion and Comprehension). Group and gender differences in ACS and WAIS-IV performance were assessed, as well as relationships between measures of alcoholism severity and social cognitive functioning.
Results
Alcoholics performed significantly worse than nonalcoholics on the ACS measures of Affect Naming and Faces Content. Alcoholic men were impaired relative to alcoholic women on Prosody Face Matching and Faces Content scores. Among alcoholics, longer durations of heavy drinking were associated with poorer performance on Affect Naming, and a greater number of daily drinks was associated with lower Prosody Face Matching performance. For alcoholic women, a longer duration of abstinence was associated with better performance on Affect Naming.
Conclusions
Alcoholic men and women showed different patterns of associations between alcoholism indices and clinically validated social cognition assessments. These findings extend into the social cognition domain, previous literature demonstrating the presence of cognitive deficits in alcoholism, their association with alcoholism severity, and variability by gender. Moreover, because impairments in social cognition can persist despite extended abstinence, they have important implications for relapse prevention.
Keywords: social cognition, emotion, alcoholism, gender, ACS
INTRODUCTION
Social cognition is broadly defined as the cognitive processes and individual factors (such as motivation and personality) involved in social interactions (Adolphs, 2003; Insel & Fernald, 2004). Elements of social cognition include emotional regulation and decision-making, and brain regions associated with these processes include frontolimbic circuitries (Adolphs, 2003; Amodio & Frith, 2006). Structural and functional neuroimaging findings have suggested that frontolimbic regions overlapping with the “social brain” are disproportionately affected in alcoholism (Moselhy, Georgiou, & Kahn, 2001; Oscar-Berman & Marinkovic, 2007). Affected regions include the orbitofrontal and anterior cingulate cortices, hippocampus, and amygdala (Makris et al., 2008; Wobrock et al., 2009), areas that also are linked to impairments in social cognition. Additionally, tractography analyses have revealed alcoholism-related abnormalities in fiber tracts (fornix and cingulum) connecting cortical and limbic portions of emotion and reward circuits (Schulte et al., 2010).
Using functional magnetic resonance imaging (fMRI), O’Daly and colleagues (2012) reported that alcoholics were impaired in recognizing expressions of fearful faces and had lower corresponding task-specific activation in the orbitofrontal cortex and insula. Marinkovic and colleagues (2009) observed dampened amygdala and hippocampal activation in alcoholics viewing positive and negative emotional facial expressions during fMRI. Maurage and colleagues (2012) found higher insula and lower frontal activation in alcoholics during social exclusion conditions of an fMRI task while alcoholics completed a game that simulated social interactions. Taken together, these fMRI results suggest that alcoholics have difficulty differentiating types of facial expressions; in turn, this might reflect broader impairments in emotional processing, as indicated by abnormal patterns of neural activity in frontolimbic brain regions.
Behavioral findings of impaired social cognitive and emotional processing in abstinent alcoholics as measured by evaluation of facial affect perception, emotional prosody, and empathy have been described by Uekermann and Daum (2008). Alcoholism-related abnormalities also have been found in cross-modal integration of social cognitive information, decoding of affective states, empathic ability, and in theory-of-mind (reviewed in Thoma et al., 2013). Maurage and colleagues (2009) reported a generalized impairment in emotional decoding and difficulty interpreting facial expressions, body postures, and emotional prosody that varied across emotional valences in alcoholics. Uekermann and colleagues (2005) showed that alcoholics were impaired in matching affective prosody to emotional facial expressions and in naming incongruent affective prosodies. Additionally, alcoholics have demonstrated high levels of alexithymia, an emotional processing deficit characterized by difficulties identifying, differentiating, and expressing feelings (Stasiewicz et al., 2012).
Social cognition has been evaluated by employing measures of affect recognition, social perception and judgment, and stimulus-relevant aspects of emotional processing and memory (Holdnack & Drozdick, 2009; Holdnack et al., 2011; Kandalaft et al., 2012). Examinations of the factor structure of the Wechsler Adult Intelligence Scale-III (WAIS; Wechsler, 1997) revealed a social cognition factor within the broader domain of intellectual functioning in healthy control (Allen & Barchard, 2009) and clinical populations (Allen et al., 2007; Goldstein et al., 2008). Abstinent alcoholics performed worse on WAIS subtests concerned with social cognition such as Comprehension and Picture Arrangement (Oscar-Berman et al., 2009), Picture Completion (reviewed in Parsons & Farr, 1981), and Object Assembly (Beatty et al., 1996).
In the present study, we assessed social cognition in abstinent long-term alcoholic and nonalcoholic control men and women using recently developed measures of social cognition from the Wechsler Advanced Clinical Solutions (ACS) module (Pearson, 2009): (a) Social Perception subtests of Affect Naming, Prosody-Face Matching, and Prosody-Pair Matching, and (b) the composite measures Faces Content and Faces Spatial (derived from the Faces I and II subtests). The ACS was developed in regard to the increasing prevalence of clinical disorders with social processing deficits and to enhance the clinical utility of intelligence and memory tests. Although ACS validation studies have examined social cognition in relation to general cognitive ability (IQ, WAIS-IV; Wechsler, 2008) and memory functioning (Wechsler Memory Scale, WMS-IV; Wechsler, 2009), there have been few examinations involving the ACS in clinical populations e.g., autism (Holdnack et al., 2011; Kandalaft et al., 2012), schizophrenia (Kandalaft et al., 2012), and Alzheimer’s disease (Holdnack & Drozdick, 2009). We know of only one study examining ACS performance in relation to alcoholism (Luhar et al., 2013).
The first aim of this study was to expand the clinical utility of the ACS by examining social cognition performance among individuals with a history of long-term alcoholism. Additionally, because there exists a wealth of evidence suggesting that alcoholism may impact brain structure and functioning differentially by gender (Ruiz et al., 2013; Ruiz & Oscar-Berman, 2013), we sought to characterize how social cognition may be variably impacted by alcoholism in men and women. As a corollary, if these deficits are present and detectable, we sought to determine how specific they are in relation to intelligence tests that contain a social cognition component (i.e., Picture Completion and Comprehension WAIS-IV subtests). We expected that alcoholics would perform more poorly on ACS measures than nonalcoholics, and that scores would differ for alcoholic men and women. The second aim of this study was to determine to what extent the quality of social cognition is related to severity indices for alcoholism, and how this effect differs between genders. We expected that higher levels of alcoholism severity would be associated with more impaired performance on ACS and intelligence tests, longer durations of abstinence would be associated with better performance, and that the pattern of associations would differ between men and women.
MATERIALS AND METHODS
Participants
Participants included 77 abstinent long-term alcoholics (37 women) and 59 demographically similar nonalcoholic controls (29 women) (Table 1). All were right-handed English speakers recruited through flyers placed in treatment facilities and in public places (e.g., churches, stores), and advertisements in newspapers and web sites. Selection procedures for both groups included an initial telephone interview to determine age, education, health history, and history of alcohol and drug use. Eligible individuals were invited to the laboratory for further screening and evaluations. This study was reviewed and approved by the Institutional Review Boards of our affiliated institutions. Participants gave informed consent for participation, and were compensated for their time.
Table 1.
Participant demographics, drinking history, and neuropsychological performance by Group and Gender. All neuropsychological test scores are scaled scores. Quantity Frequency Index scores are roughly equivalent to the average number of drinks consumed per day (one drink is roughly equivalent to one ounce of ethanol) by accounting for the number of ounces of beverage consumed and the number of ounces of ethanol included in each type of alcoholic beverage (wine, beer and liquor) during the last six months for nonalcoholics, and approximately for the last six months of drinking for alcoholics.
| All Alcoholics |
All Nonalcoholics |
Alcoholic Women |
Alcoholic Men |
Nonalcoholic Women |
Nonalcoholic Men |
|
|---|---|---|---|---|---|---|
| n = 77 | n = 59 | n = 37 | n = 40 | n = 29 | n = 30 | |
| Education (years) | ||||||
| Mean | 14.47 | 15.02 | 14.80 | 14.18 | 14.90 | 15.13 |
| SD | 2.09 | 2.45 | 1.97 | 2.18 | 2.51 | 2.42 |
| Range | 11–20 | 10–20 | 12–19 | 11–20 | 12–20 | 10–20 |
| Age (years) | ||||||
| Mean | 51.81 | 52.74 | 53.28 | 50.44 | 55.39 | 50.18 |
| SD | 11.16 | 13.40 | 11.85 | 10.45 | 13.60 | 12.92 |
| Range | 28.08–76.75 | 26.58–81.58 | 31.00–75.58 | 28.08–76.75 | 30.50–78.33 | 26.58–81.58 |
|
WAIS-IV Full Scale IQ†,a,k |
||||||
| Mean | 103.04 | 108.17 | 102.24 | 103.78 | 106.55 | 109.73 |
| SD | 15.31 | 14.08 | 13.79 | 16.73 | 14.51 | 13.71 |
| Range | 75–142 | 79–141 | 75–132 | 75–142 | 79–141 | 89–141 |
|
WMS-IV Delayed Memory Index Score‡a,e,i |
||||||
| Mean | 105.40 | 112.62 | 107.17 | 103.76 | 114.21 | 111.03 |
| SD | 16.93 | 14.30 | 19.42 | 14.33 | 12.55 | 15.93 |
| Range | 62–135 | 78–146 | 62–135 | 76–133 | 86–146 | 78–137 |
|
Hamilton Rating Scale for Depressionb,d,f,l |
||||||
| Mean | 3.17 | 1.58 | 3.70 | 2.68 | 2.03 | 1.13 |
| SD | 3.42 | 2.17 | 3.31 | 3.49 | 1.97 | 2.29 |
| Range | 0–14 | 0–8 | 0–10 | 0–14 | 0–7 | 0–8 |
|
Duration of Heavy Drinking (years)b,d,f,g,j,l |
||||||
| Mean | 15.51 | 0.00 | 13.12 | 17.73 | 0.00 | 0.00 |
| SD | 7.91 | 0.00 | 5.66 | 9.06 | 0.00 | 0.00 |
| Range | 5–37 | 0.00 | 5–25 | 5–37 | 0.00 | 0.00 |
|
Quantity Frequency Index (drinks per day)b,d,f,g,j,l |
||||||
| Mean | 11.52 | 0.19 | 8.89 | 13.96 | 0.23 | 0.15 |
| SD | 8.23 | 0.29 | 6.91 | 8.67 | 0.36 | 0.18 |
| Range | 2.54–44.16 | 0.00–1.42 | 2.54–34.82 | 3.03–44.16 | 0.00–1.42 | 0.00–0.73 |
|
Length of Abstinence (years)^b,d,h,l |
||||||
| Mean | 6.64 | 2.67 | 9.96 | 3.57 | 2.96 | 2.39 |
| SD | 9.43 | 8.14 | 11.66 | 5.28 | 8.03 | 8.39 |
| Range | 0.10–36.14 | 0.002–40.20 | 0.10–36.14 | 0.10–27.43 | 0.005–31.87 | 0.003–40.19 |
| Number of Individuals Having Engaged in Treatment |
69 | N/A | 32 | 37 | N/A | N/A |
| Number of Individuals Meeting DSM-IV Criteria for Current Nicotine Dependence+ |
7 | 1 | 4 | 3 | 0 | 1 |
For one alcoholic woman, IQ was estimated from a test of vocabulary (premorbid intelligence).
Four alcoholics (2 women) and one nonalcoholic man had missing Delayed Memory Index Scores.
Four nonalcoholics (2 women) had missing LOA measures because they never drank.
Three alcoholics (2 women) had missing data for DSM-IV Criteria for Nicotine Dependence.
Significant Differences (p ≤ .05):
Alcoholics < Nonalcoholics;
Alcoholics > Nonalcoholics;
Alcoholic Women < Nonalcoholic Women;
Alcoholic Women > Nonalcoholic Women;
Alcoholic Men < Nonalcoholic Men;
Alcoholic Men > Nonalcoholic Men;
Alcoholic Women <Alcoholic Men;
Alcoholic Women > Alcoholic Men;
Alcoholic Men < Nonalcoholic Women;
Alcoholic Men > Nonalcoholic Women;
Alcoholic Women < Nonalcoholic Men;
Alcoholic Women > Nonalcoholic Men.
Assessments
Participants underwent a medical history interview and vision testing, responded to questionnaires, and completed a computer-assisted, shortened version of the Computerized Diagnostic Interview Schedule (DIS; Robins et al., 1989, Robins et al., 2000), which provides lifetime psychiatric diagnoses according to American Psychiatric Association DSM-III-R or DSM-IV-TR criteria (American Psychiatric Association, 1997, 2000). Participants were excluded from further participation if any source (DIS scores, hospital records, referrals, or personal interviews) indicated that English was not one of their first languages, or if they had one of the following: Korsakoff’s syndrome; HIV; diagnosed hepatic disease (including cirrhosis); major head injury with loss of consciousness greater than 15 minutes; stroke; seizures unrelated to alcoholism; schizophrenia; Hamilton (1960) depression score over 16; corrected visual acuity worse than 20/50 in both eyes; or any illicit comorbid drug use (except marijuana) more frequently than once per week within the four years prior to enrollment (four alcoholic participants used marijuana more than once per week within the four years prior to enrollment).
Participants received a structured interview to assess their drinking histories (MacVane et al., 1982) including abstinence duration (LOA; length of abstinence), and duration of heavy drinking (DHD), i.e., years of consumption of 21 drinks or more per week (one drink: 355 mL beer, 148 mL wine, or 44 mL hard liquor; see Table 1). A Quantity Frequency Index score (QFI; Cahalan et al., 1969), which factors the amount, type, and frequency of alcohol usage (roughly corresponding to number of drinks per day, at approximately one ounce of ethanol per drink) over either the last six months (for nonalcoholics) or over the six months preceding cessation of drinking (for alcoholics) was calculated for each participant. Alcoholic participants endorsed whether they ever engaged in any type of treatment to aid in recovery (e.g., Alcoholics Anonymous, psychotherapy; see Table 1). The alcoholic participants met DSM-III-R or DSM-IV-TR criteria for alcohol abuse or dependence, drank heavily for at least five years in their lives, and had abstained from alcohol for at least four weeks prior to testing.
Psychological measures included the ACS module (Pearson, 2009) developed to assess social cognition skills in clinical populations and to enhance clinical utility of the intelligence tests (WAIS-IV) and memory tests (WMS-IV), which also were administered. Measures of performance were analyzed using age-corrected scaled scores. Participants completed procedures across two or three sessions that lasted up to three hours each. Psychometric properties of the assessment instruments are available in the referenced citations for each of the standardized tests administered. Normative data have been established among alcoholic participants for intelligence and memory tests, but we know of no ACS normative data for an alcoholic population.
Included in the assessment were two intelligence subtests of the WAIS-IV containing a social cognition component (i.e., Picture Completion and Comprehension), to determine how performance on ACS relates to the intelligence subtests. Picture Completion requires identification of a missing part of everyday scenes within a time limit, and measures: (1) ability to quickly perceive visual details, (2) relate details to a daily routine, (3) perceptual organization, and (4) visual processing. Comprehension requires answering questions about social conventions, and measures abstract reasoning ability concerning social rules, judgment, and expressions. We measured social cognition using the Social Perception and Faces subtests of the ACS module. The tests of Social Perception, which assess comprehension of social communication, included Affect Naming, Prosody Face Matching, and Prosody Pair Matching. Affect Naming measures facial affect recognition and matching of emotion labels from photographs of faces. Participants identify the emotion expressed in the photograph from a list of seven possible emotion labels (happy, sad, angry, afraid, surprised, disgusted, or neutral). Prosody Face Matching measures affect recognition from prosody expressed during spoken sentences, and from photographs of faces. Participants match the emotion expressed in the photograph to the emotional tone expressed by the speaker in the audio file sentence. For example, a participant hears a sentence such as “That was really good” in a positive tone of voice, meant to express happiness and gratitude. A correct response requires matching the positive emotional tone to a photograph with a happy facial expression. Prosody Pair Matching measures affect recognition from prosody expressed during sentences and photographs of interacting pairs of people. Two evaluations contribute to the score: (1) matching the emotional tone of the speaker in an audio file sentence, with the facial expression and body language expressed in a photograph of interacting pairs of people, and (2) identifying the emotion expressed by the speaker and determining if their tone of voice changes the meaning of the sentence. For example, a participant hears a sentence, “Don’t work too hard” in a sarcastic tone of voice, indicating the speaker is describing someone who is lazy. The response requires matching the emotional tone of the speaker to one of the photographs of interacting pairs of people, and designating if the tone of voice matches the speaker’s intended meaning.
The Faces ACS subtests measure recognition, discrimination, and learning of 10 faces in immediate- and delayed-recall conditions. Faces Content measures learning of faces and encoding of features for immediate and long-term recall, and Faces Spatial measures immediate and long-term spatial memory of faces. The examiner shows the participant a stimulus page that consists of a grid with photographs of 10 faces for 10 seconds, and then removes it from view. The participant selects the 10 faces from a set of 20 cards (comprised of 10 targets and 10 distracters) and places the correct cards on an empty grid in the same locations as previously shown on the stimulus page. The participant must recognize the correct faces and place them in the correct locations on the grid. The participant views the same 10 faces and locations over four learning trials. The Faces Content score reflects the participant’s ability to recall the faces across four learning trials, and the Faces Spatial score reflects the participant’s ability to recall locations of the faces on the grid across four learning trials.
Statistical Analyses
All analysis of variance (ANOVA) models examining the effects of gender and group on ACS and intelligence subtests were two-tailed, thereby satisfying a conservative statistical threshold for examining performance on measures that have not yet been thoroughly investigated in alcoholism. Regression analyses testing the associations among social cognition performance and each of the drinking variables were conducted using a Bonferroni adjusted alpha level of .007 per test (.05/7). Outliers on drinking variables and on social cognition and intelligence subtests that were three standard deviations or more from the group mean were removed prior to correlation and regression analyses; no more than one outlier was observed for any of the measures. Table 2 provides means, standard deviations, and ranges of scores for each group and each gender within group.
Table 2.
ACS and Wechsler Adult Intelligence Scale (WAIS-IV) scores. All neuropsychological test scores are scaled scores.
| All Alcoholics |
All Nonalcoholics |
Alcoholic Women |
Alcoholic Men |
Nonalcoholic Women |
Nonalcoholic Men |
|
|---|---|---|---|---|---|---|
| n = 77 | n = 59 | n = 37 | n = 40 | n = 29 | n = 30 | |
| Affect Naming | ||||||
| Mean | 10.08 | 11.19 | 10.51 | 9.68 | 11.52 | 10.87 |
| SD | 2.64 | 2.87 | 2.38 | 2.83 | 3.15 | 2.60 |
| Range | 4–15 | 3–18 | 6–15 | 4–15 | 5–18 | 3–16 |
|
Prosody Face Matching |
||||||
| Mean | 10.69 | 11.19 | 11.51 | 9.93 | 11.28 | 11.10 |
| SD | 2.88 | 2.90 | 2.72 | 2.84 | 3.13 | 2.72 |
| Range | 3–19 | 5–19 | 5–19 | 3–15 | 5–19 | 5–16 |
|
Prosody Pair Matching |
||||||
| Mean | 10.69 | 10.78 | 11.35 | 10.08 | 10.79 | 10.77 |
| SD | 2.97 | 3.18 | 2.87 | 2.96 | 2.90 | 3.49 |
| Range | 4–17 | 2–18 | 5–16 | 4–17 | 5–18 | 2–17 |
| Faces Content^ | ||||||
| Mean | 8.76 | 10.05 | 9.62 | 7.95 | 10.14 | 9.97 |
| SD | 3.49 | 3.35 | 3.53 | 3.29 | 3.67 | 3.07 |
| Range | 2–16 | 4–18 | 2–16 | 2–16 | 4–18 | 5–18 |
| Faces Spatial^ | ||||||
| Mean | 8.58 | 9.24 | 8.78 | 8.38 | 8.76 | 9.70 |
| SD | 2.65 | 2.72 | 2.36 | 2.92 | 2.95 | 2.45 |
| Range | 2–14 | 4–14 | 5–13 | 2–14 | 4–14 | 5–14 |
| WAIS-IV Comprehension | ||||||
| Mean | 11.47 | 12.12 | 11.11 | 11.80 | 11.72 | 12.50 |
| SD | 3.33 | 2.78 | 3.60 | 3.07 | 2.70 | 2.85 |
| Range | 4–19 | 5–19 | 4–19 | 6–18 | 8–18 | 5–19 |
|
WAIS-IV Picture Completion |
||||||
| Mean | 9.81 | 10.10 | 10.00 | 9.63 | 10.21 | 10.00 |
| SD | 2.97 | 2.83 | 3.10 | 2.88 | 2.93 | 2.77 |
| Range | 2–16 | 3–15 | 4–16 | 2–16 | 3–15 | 4–15 |
One alcoholic man was missing scores for Faces Content and Faces Spatial.
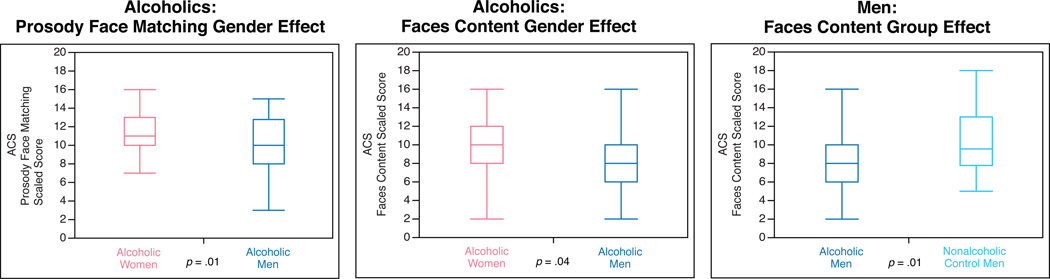
Because the impact of alcoholism has been shown to differ for men and women in other domains (Ruiz & Oscar-Berman, 2013), we sought to characterize the influence of alcoholism on social cognition for each gender separately. To this end, we used separate one-way ANOVA models to test the simple main effect of alcoholism Group within each gender and the simple main effect of Gender within each group on social cognition and intelligence test scores (Table 3, Figure 1).
Table 3.
Simple main effects within each Group and Gender on ACS and Wechsler Adult Intelligence Scale (WAIS-IV) scores. F-Values for ANOVA models indicating simple main effects are shown for each group and gender.
| Alcoholics | Nonalcoholics | Men | Women | |
|---|---|---|---|---|
| Main Effect | Gender | Gender | Group | Group |
| n = 77 | n = 59 | n = 70 | n = 66 | |
| Affect Naming | 1.96 | 0.75 | 3.26+ | 2.18 |
| Prosody Face Matching | 6.25* | 0.05 | 3.04+ | 0.11 |
| Prosody Pair Matching | 3.69+ | 0.001 | 0.80 | 0.61 |
| Faces Content^ | 4.57* | 0.04 | 6.76* | 0.34 |
| Faces Spatial^ | 0.43 | 1.78 | 3.95+ | 0.002 |
| WAIS-IV: Picture Completion |
0.30 | 0.08 | 0.30 | 0.08 |
| WAIS-IV: Comprehension |
0.83 | 1.15 | 0.95 | 0.59 |
p ≤ .05;
.05 < p < .10.
One alcoholic man was missing scores for Faces Content and Faces Spatial.
Figure 1.
Significant Group and Gender simple main effects from ANOVA analyses. Alcoholic women performed better than alcoholic men on Prosody Face Matching and Faces Content. Nonalcoholic control men outperformed alcoholic men on Faces Content. F-values are reported in Table 3. Box boundaries indicate interquartile range (IQR). Median scores are indicated by the lines within the boxes. Whiskers indicate the furthest points within 1.5 × IQR from the boxes.
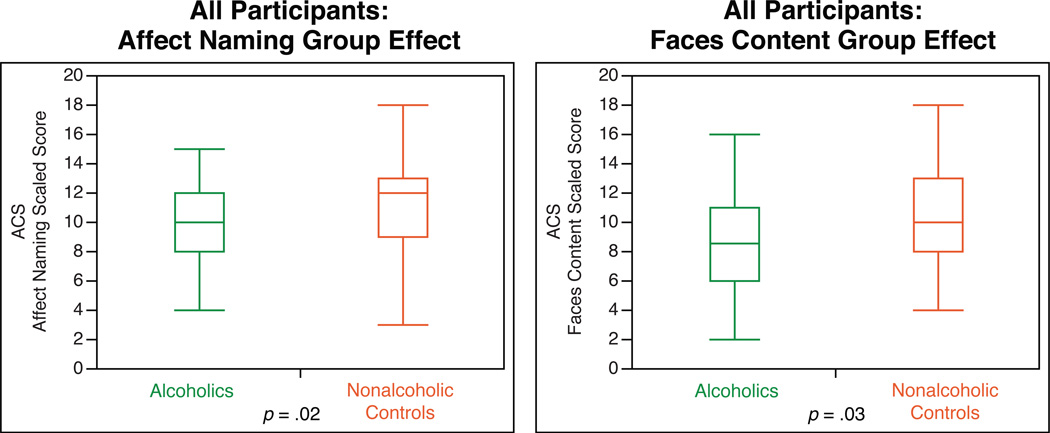
To determine whether the impact of alcoholism on ACS and intelligence scores were significantly different for men and women, we tested the Group by Gender interaction in a two-way ANOVA model (Table 4, Figure 2). Analysis of the combined gender group allowed us to test our group-level hypotheses with greater statistical power, and to highlight how reports from gender-mixed groups of alcoholics might be masking effects specific to (or stronger in) a single gender.
Table 4.
Main effects of Group and Gender on neuropsychological performance. F-Values for ANOVA models indicating main effects of Group and Gender on ACS and Wechsler Adult Intelligence Scale (WAIS-IV) scores are shown for all participants. For ANOVAs that included the Group by Gender interaction, no interactions were significant.
| All Participants n = 136 |
||
|---|---|---|
| Main Effect | Group | Gender |
| Affect Naming | 5.44* | 2.62 |
| Prosody Face Matching |
0.97 | 3.95* |
| Prosody Pair Matching |
0.03 | 1.96 |
| Faces Content^ | 4.71* | 3.01 |
| Faces Spatial^ | 2.00 | 0.16 |
| WAIS-IV: Picture Completion |
0.34 | 0.36 |
| WAIS-IV: Comprehension |
1.52 | 1.89 |
p < .05.
One alcoholic man was missing scores for Faces Content and Faces Spatial.
Figure 2.
Significant Group main effects from ANOVA analyses. Alcoholics showed impaired performance on Affect Naming and Faces Content. F-values for the full model are shown in Table 4. Box boundaries indicate interquartile range (IQR). Median scores are indicated by the lines within the boxes. Whiskers indicate the furthest points within 1.5 × IQR from the boxes.
Next, to determine the extent to which the ACS measures were associated with scores on the intelligence subtests, pairwise Pearson correlations were conducted between each of the five social cognition variables with Picture Completion and Comprehension (Table 5).
Table 5.
Correlations of ACS measures with Wechsler Adult Intelligence Scale (WAIS-IV) subtest scores are shown for all participants.
| WAIS-IV: Picture Completion |
WAIS-IV: Comprehension | |
|---|---|---|
| Affect Naming | 0.25** | 0.31*** |
| Prosody Face Matching | 0.34*** | 0.44*** |
| Prosody Pair Matching | 0.39*** | 0.46*** |
| Faces Content^ | 0.12 | 0.26** |
| Faces Spatial^ | 0.15+ | 0.23*** |
p ≤ .001;
p ≤ .01;
.05 < p < .10.
One alcoholic man was missing scores for Faces Content and Faces Spatial.
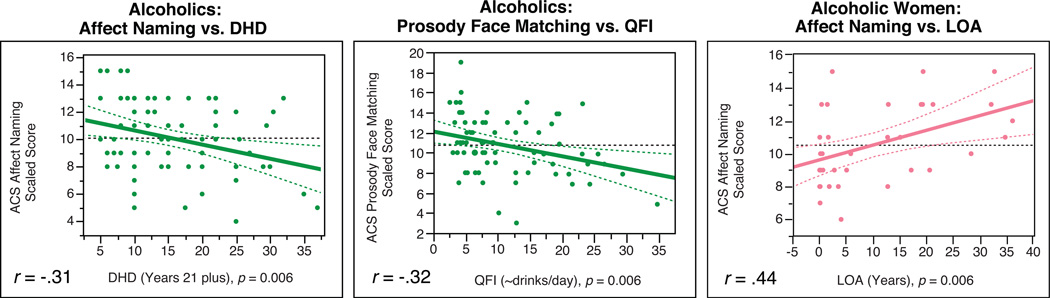
To evaluate the impact of alcoholism severity, we examined associations of social cognition and intelligence subtests with each of the drinking indices (DHD, QFI, and LOA), by calculating pairwise Pearson correlations (two-tailed) in the alcoholics (Figure 3). Because alcoholic men and women differed on the drinking variables, we also examined these correlations for each gender separately. Finally, we examined the interactions of Gender with each of the drinking variables in a multiple regression model to determine if the relationships were significantly stronger for men or women.
Figure 3.
Pairwise correlations between each of the drinking variables (DHD, QFI, and LOA) and each of the social cognition measures for the alcoholic participants. Significant inverse correlations between DHD and Affect Naming were identified among alcoholics. Significant inverse correlations between QFI and Prosody Face Matching were identified among alcoholics. Alcoholic women showed positive correlations between LOA and Affect Naming. Dashed lines indicate means and the 95% confidence interval of the slope.
RESULTS
Participants
Alcoholic and nonalcoholic groups were of similar ages and education levels (Table 1). The alcoholics had significantly lower Full Scale IQ and Delayed Memory Index scores, and higher Hamilton depression scores than nonalcoholics (Table 1). Alcoholic men had a significantly longer duration of heavy drinking, and drank significantly more drinks than alcoholic women, and alcoholic women had a significantly longer length of abstinence than alcoholic men. Assessments among the drinking measures (DHD, QFI, LOA) showed that DHD was positively correlated with QFI (r = 0.25, p < .05) and negatively correlated with LOA (r = −0.23, p < .05). An inverse correlation between QFI and LOA was identified at a trend level (r = −0.22, .05 < p < .10).
Group and Gender in Relation to Social Cognition Measures
Means, standard deviations, and ranges of these measures are reported in Table 2. Simple main effects ANOVA results examining Group effects within each Gender, and Gender effects within each Group, are reported in Table 3 and are shown in Figure 1. Analyses revealed that alcoholic women significantly outperformed alcoholic men on Prosody Face Matching (F(1, 76) = 6.25, p = .01, η2 = .08) and on Faces Content (F(1, 75) = 4.57, p = .04, η2 = .04). Among male participants, alcoholic men performed significantly worse than nonalcoholic men on Faces Content (F(1, 68) = 6.76, p = .01, η2 = .09). There were no significant differences between alcoholic and nonalcoholic women on any of the five ACS variables or the Picture Completion and Comprehension subtests.
Two-way between-groups ANOVAs examined the interaction of Gender and Group on ACS and intelligence subtest scores (Table 4, Figure 2). None were significant for any of the ACS scores, nor the intelligence subtests. However, in the model including both factors (Group and Gender), there were significant main effects of Group for Affect Naming (F(2, 135) = 5.34, p = .02, η2 = .06) and Faces Content (F(2, 134) = 4.72, p = .03, η2 = .04), wherein alcoholics scored lower than controls. For Prosody Face Matching, there was a significant main effect Gender, (F(2, 135) = 3.95, p = .05, η2 = .04), suggesting that women performed better than men on this measure. There were no significant Gender or Group differences for the Picture Completion or Comprehension subtests.
Associations among Social Cognition Variables
Table 5 summarizes correlations between the five ACS measures and the two intelligence subtests that are concerned with social cognition. Among all participants, the five ACS measures correlated significantly with Comprehension scores, and four of five did so with Picture Completion (range of coefficients was .23 to .46).
Associations between Alcoholism Variables and Social Cognition Measures
Pairwise correlations between each of the drinking variables and each of the social cognition measures among the alcoholic participants were calculated. Because significant differences existed in drinking history between alcoholic men and women, we also conducted analyses calculating Pearson correlation coefficients for these relationships within each gender separately (Figure 3). After applying a Bonferroni adjusted alpha level of .007, DHD was significantly negatively correlated with Affect Naming scores (r = −0.31, p =.006). There were trend-level negative correlations of DHD with Prosody Face Matching (r = −0.24, p = .04), and with spatial memory for faces among alcoholic women in particular (r = −0.38, p =.02). QFI was significantly negatively correlated with Prosody Face Matching scores (r = −0.32, p =.006). There was a significant interaction between QFI and Gender for Affect Naming scores (F(3, 74) = 9.01, p = .004, η2 =.14), indicating a positive correlation for alcoholic men and a negative correlation for alcoholic women, although separate correlations for each gender were not statistically significant. LOA was significantly associated with higher scores on Affect Naming (r = 0.44, p =.006) for alcoholic women only. Other effects emerged as trends, consistently suggesting improved performance with increased abstinence. That is, for alcoholics, positive correlations emerged between LOA and Affect Naming (r = 0.27, p = .02), and between LOA and Prosody Face Matching (r = 0.23, p = .05). When considering alcoholic women, LOA was positively associated with Picture Completion scores (r = 0.36, p = .03). The interaction effect of LOA and Gender for Faces Spatial (F(3, 73) = 3.58, p = .06, η2 =.07) also emerged as a trend, suggesting that among alcoholic men, higher scores on Faces Spatial were associated with longer periods of abstinence (r = 0.32, p = .05), but not among alcoholic women (r = 0.05, p = .76).
DISCUSSION
In this study, we expected that participants with a history of alcoholism would have impaired social cognition performance, and that gender disparities in the social cognition domain would be seen in association with alcoholism. We found that alcoholic men performed worse than nonalcoholic men on Faces Content, an impairment that was not identified for women. Further, alcoholic men performed worse than alcoholic women on both Prosody Face Matching and Faces Content. When genders were combined, we found that the long-term abstinent alcoholic participants scored significantly worse than nonalcoholic participants on Affect Naming and Faces Content.
We also expected that higher levels of alcoholism severity, as signified by duration of drinking and amount of alcohol consumed, would be associated with poorer performance on social cognition measures, and longer durations of abstinence would be associated with better performance. Inverse correlations of alcoholism duration with specific social cognition scores confirmed our hypotheses: (1) longer periods of heavy drinking were associated with impaired ability to identify facial emotions, and (2), larger quantities of drinks consumed per day were associated with impaired ability to match emotional tone to facial expressions. Analyses of the effect of abstinence duration suggested that among alcoholic women, identification of emotional facial expressions improved with abstinence.
These findings extend into the social cognition domain, previous literature demonstrating that cognitive deficits in alcoholism are associated with increased alcoholism severity (Oscar-Berman & Marinkovic, 2007; Oscar-Berman et al., 2014). The ACS measures of social cognition were developed to refine clinical assessments of social cognitive abnormalities, but these measures have not been employed in research with alcoholic participants. Instead, prior studies of socially relevant abnormalities with this population have included a variety of procedures that had not yet been validated on clinical populations, e.g., laboratory-based probes of emotional facial perception and recognition (Clark et al., 2007; Oscar-Berman et al., 1990). Thus, the results of the present study expand findings of emotional facial expression identification and psychosocial abnormalities in alcoholism (Luhar et al., 2013) by using a novel standardized battery—the ACS.
Alcoholism and Social Cognition Measures
Participants with an alcohol history performed significantly worse than nonalcoholic controls on the ACS subtests of Affect Naming and Faces Content. Affect Naming measures the basic ability to discriminate among emotional facial expressions and correctly label emotions, and Faces Content measures the basic ability to encode and remember facial features for immediate and long-term recall. Deficits in these measures may reflect impairments of fundamental aspects of social cognition (i.e., facial affect perception and facial identification), separately from presumptive higher-level appreciation of nuance and judgment, as measured by the Picture Completion and Comprehension subtests, where no deficits were observed. The observed deficiencies in Affect Naming and Faces Content are especially important because impairments in evaluating emotional facial expressions have been shown to be related to interpersonal problems and may be tied to relapse risk (Kornreich et al., 2002). Moreover, social learning and social control skills have been associated with reduced relapse rates and better long-term outcomes following detoxification (Moos & Moos, 2007)
Successful performance on Affect Naming requires an understanding and foundational knowledge of emotional facial expressions without any additional information to identify the correct emotional label, as well as the integration of verbal knowledge for emotional labels and visual discrimination of facial expressions. Successful performance on Faces Content requires an ability to correctly encode facial features and to recall facial identification across learning trials. Our findings converge with previous reports of diminished social cognitive functioning in alcoholism manifested by impairments in facial affect perception and facial identification (Clark et al., 2007; Foisy et al., 2007; Marinkovic et al., 2009). In particular, our Affect Naming findings align with previous studies showing social cognition deficits in alcoholism, such as evaluating and labeling affect from pictures of faces (Uekermann & Daum, 2008; Clark et al., 2007), and that these deficits are particularly related to a history of heavy drinking (Sorocco et al., 2010). Our Faces Content findings also support previous studies demonstrating visual processing deficits in alcoholism (Beatty et al., 1996) and memory disturbances for facial identification tasks in particular (Oscar-Berman et al., 1990).
It has been suggested that compensation (reorganization) of brain function occurs after years of abstinence by recruitment of new and/or additional neural networks following alcoholism-related brain damage (Chanraud et al., 2013; Oscar-Berman & Marinkovic, 2007; Sullivan & Pfefferbaum, 2005). Since our sample of abstinent alcoholic participants had more than six years of abstinence on average, it is possible that recovery of functions occurred earlier in the course of sobriety, rendering group differences less detectable. In our sample, there was a suggestion that performance on most social cognition measures improved with abstinence, with the notable exception of Faces Content, on which alcoholism-related deficits remained. Length of abstinence was significantly associated with improved Affect Naming scores for alcoholic women. Deficits in Affect Naming were identified despite better performance being associated with abstinence, suggesting that group differences in identifying emotions were even more pronounced earlier in recovery.
Previous studies have shown associations between social competence and performance on several intelligence subtests such as Comprehension, Picture Arrangement (Oscar-Berman et al., 2009), Picture Completion (reviewed in Parsons & Farr, 1981), and Object Assembly (Beatty et al., 1996). In the present study, the mean sobriety duration of our alcoholic participants was more than six years, which might account for the absence of clear deficits on the Picture Completion and Comprehension subtests of the WAIS-IV. Abstinence has been identified as an important predictor of alcoholism-related cognitive deficits (Fein et al., 1990), and in the present sample, Picture Completion scores were positively associated with abstinence duration among alcoholic women.
Gender and Social Cognition Measures
Consistent with gender-based performance expectations (Rosip & Hall, 2004), we found that women performed better than men on Prosody Face Matching. We expected that the impact of alcoholism on social cognition would differ between genders. In general, the social cognition performance by alcoholic men was below that of other groups. Alcoholic men showed significant or trend-level deficits on four of the five social cognition measures relative to nonalcoholic men, whereas no such deficits were identified among alcoholic women. Compared to alcoholic women, the alcoholic men had on average an additional 4.5 years of heavy drinking, drank approximately five additional drinks per day, and were sober for an average of six fewer years. Although the men tended to have more severe measures on these indices, for physiological reasons, we might not expect that each of these variables would impact men and women in the same way (Ruiz & Oscar-Berman, 2013).
While a comparable impact of years of heavy drinking was found between alcoholic men and women on Affect Naming scores, only alcoholic women had a clearly positive association between abstinence duration and emotional identification ability. Alcoholic women tended to have a stronger negative association between duration of heavy drinking and Faces Spatial scores than alcoholic men. In contrast, alcoholic men showed evidence for recovery of spatial memory for faces, whereas alcoholic women did not. These findings are consistent with previous research demonstrating that alcoholic women showed less evidence of recovery in visuospatial processing relative to other cognitive domains (Sullivan et al., 2002).
Previous studies using emotional faces and voices have reported deficits in processing (Kornreich et al., 2013; Monnot et al., 2001) that suggested alcoholism-related cross-modal difficulties for evaluation of socially-relevant stimuli (Maurage et al., 2007; Maurage et al., 2013). Although we did not observe significant differences between the alcoholic and control groups on measures of emotional prosody perception (i.e., Prosody Face Matching and Prosody Pair Matching), we did find that alcoholic men were impaired relative to alcoholic women, and the alcoholic men had lower scores than the nonalcoholic men on Prosody Face Matching. This gender difference in emotional prosody ability among alcoholics may be due partially to the disparity in number of daily drinks consumed between the men and women, and a greater number of drinks was associated with poorer Prosody Face Matching scores.
Additionally, alcoholic women showed a trend-level association between length of abstinence and Prosody Face Matching, further contributing to the notion that gender differentially influences the social cognition deficits observed in conjunction with alcoholism.
Limitations
In this study, the alcoholic participants reported significantly higher levels of depression as measured by the Hamilton Rating Scale for Depression, although neither group had any Hamilton scores above the clinical threshold for depression. Nevertheless, when we used multiple regression analyses to examine the influence of Hamilton scores on the measures of social cognition, we found no statistically significant effects. While the Hamilton itself was not a significant predictor, the positive correlation between number of daily drinks and Affect Naming scores among alcoholic men was eliminated when Hamilton scores were included as a covariate. Additionally, the alcoholic participants were more likely than nonalcoholic participants to be currently taking antidepressants (alcoholics n = 17; nonalcoholics n = 1). Examination of the influence of antidepressant medications on measures of social cognition using multiple regression revealed no significant effects except on Affect Naming, where antidepressant medication usage was associated with higher scores. Deficits in emotion perception and regulation in abstinent alcoholics may be indicative of underlying abnormalities in brain regions known to be associated with alcoholism. However, the present study did not include direct measures of brain defects in association with alcoholism. Finally, because our sample of alcoholics had a mean sobriety duration of more than six years, our findings regarding the clinical utility of the ACS for an alcoholic population should be interpreted in the context of an extended duration of abstinence.
Future Directions and Clinical Implications
Brain volumetric measures of regions known to underlie social cognition have not been evaluated extensively in relation to alcoholism. Therefore, future characterizations of the neuroanatomical architecture associated with social cognition can help determine how alcoholism-related abnormalities in these neural systems are associated with underlying emotional difficulties, executive dysfunction, and addictions. From a clinical perspective, further analyses of the influence of alcoholism-related impairments in social cognition could inform treatment strategies for reversal of compromised interpersonal functioning, adherence to conditional social rules, and improvements in response regulation and emotional control.
Acknowledgements
This research was supported by grants from the National Institutes of Health, NIAAA R01-AA07112 and K05-AA00219, and the Medical Research Service of the US Department of Veterans Affairs.
Contributor Information
Mary M. Valmas, Department of Psychology, Suffolk University, Department of Anatomy & Neurobiology, Boston, University School of Medicine, and Research Service, Boston VA Healthcare System
Susan Mosher Ruiz, Department of Anatomy & Neurobiology, Boston University School of Medicine, and Research Service, Boston VA Healthcare System
David A. Gansler, Department of Psychology, Suffolk University
Kayle S. Sawyer, Department of Anatomy & Neurobiology, Boston University School of Medicine, and Research Service, Boston VA Healthcare System
Marlene Oscar-Berman, Departments of Psychiatry, Neurology, and Anatomy & Neurobiology, Boston University School of Medicine, and Research Service, Boston VA Healthcare System
REFERENCES
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Allen DN, Barchard KA. Identification of a social cognition construct for the WAIS-III. Applied Neuropsychology. 2009;16:262–274. doi: 10.1080/09084280903297727. [DOI] [PubMed] [Google Scholar]
- Allen DN, Strauss GP, Donohue B, van Kammen DP. Factor analytic support for social cognition as a separable cognitive domain in schizophrenia. Schizophrenia Research. 2007;93:325–333. doi: 10.1016/j.schres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-III-R. Washington, D.C.: American Psychiatric Association; 1987. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. Journal of Studies on Alcohol and Drugs. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. American drinking practices: A national study of drinking behavior and attitudes (Monograph No. 6) New Brunswick, NJ: Rutgers Center for Alcohol Studies; 1969. [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cerebral Cortex. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. The Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Petiau C, Parez A, Hanak C, Verbanck P, Pelc I, Philippot P. Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatry Research. 2007;150:33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Allen DN, Minshew NJ, Williams DL, Volkmar F, Klin A, Schultz RT. The structure of intelligence in children and adults with high functioning autism. Neuropsychology. 2008;22:301–312. doi: 10.1037/0894-4105.22.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MA. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack J, Drozdick L. Advanced Clinical Solutions Clinical and Interpretive Manual, in Series Advanced Clinical Solutions Clinical and Interpretive Manual. San Antonio, TX: Pearson Assessments; 2009. [Google Scholar]
- Holdnack J, Goldstein G, Drozdick L. Social perception and WAIS-IV Performance in adolescents and adults diagnosed with Asperger's Syndrome and Autism. Assessment. 2011;18:192–200. doi: 10.1177/1073191110394771. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kandalaft MR, Didehbani N, Cullum CM, Krawczyk DC, Allen TT, Tamminga CA, Chapman SB. The Wechsler ACS social perception subtest a preliminary comparison with other measures of social cognition. Journal of Psychoeducational Assessment. 2012;30:455–465. [Google Scholar]
- Kornreich C, Brevers D, Canivet D, Ermer E, Naranjo C, Constant E, Verbanck P, Campanella S, Noel X. Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addiction. 2013;108:80–88. doi: 10.1111/j.1360-0443.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol and Alcoholism. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Luhar RB, Sawyer KS, Gravitz Z, Ruiz SM, Oscar-Berman M. Brain volumes and neuropsychological performance are related to current smoking and alcoholism history. Neuropsychiatric Disease and Treatment. 2013;9:1767–1784. doi: 10.2147/NDT.S52298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVane J, Butters N, Montgomery K, Farber J. Cognitive functioning in men social drinkers; a replication study. Journal of Studies on Alcohol and Drugs. 1982;43:81–95. doi: 10.15288/jsa.1982.43.81. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism: Clinical & Experimental Research. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Charest I, Martin S, de Timary P. Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol and Alcoholism. 2009;44:476–485. doi: 10.1093/alcalc/agp037. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Pham TH, Joassin F. The crossmodal facilitation effect is disrupted in alcoholism: A study with emotional stimuli. Alcohol and Alcoholism. 2007;42:552–559. doi: 10.1093/alcalc/agm134. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Pesenti M, Grandin C, Heeren A, Philippot P, de Timary P. The neural network sustaining crossmodal integration is impaired in alcohol-dependence: an fMRI study. Cortex. 2013;49:1610–1626. doi: 10.1016/j.cortex.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Protective resources and long-term recovery from alcohol use disorders. Drug and Alcohol Dependence. 2007;86:46–54. doi: 10.1016/j.drugalcdep.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol and Alcoholism. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- O'Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N, Weber DA. Emotional perception and memory in alcoholism and aging. Alcoholism: Clinical and Experimental Research. 1990;14:383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas M, Sawyer K, Ruiz S, Luhar R, Gravitz Z. Profiles of impaired, spared, and recovered neuropsychological processes in alcoholism. 1st ed. Edinburgh: Elsevier; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Kirkley SM, Gansler DA, Merritt D, Couture A. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatric Disease and Treatment. 2009;5:309–326. doi: 10.2147/ndt.s4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons O, Farr S. The neuropsychology of alcohol and drug use. Handbook of Clinical Neuropsychology. 1981;1:320–365. [Google Scholar]
- Pearson N. Advanced clinical solutions for WAIS-IV and WMS-IV: Administration and scoring manual. San Antonio: The Psychological Corporation; 2009. [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W, North C, Rourke K. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) in Series Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 2000. [Google Scholar]
- Robins L, Helzer J, Cottler L, Goldring E. NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R) 1989 [Google Scholar]
- Rosip JC, Hall JA. Knowledge of nonverbal cues, gender, and nonverbal decoding accuracy. Journal of Nonverbal Behavior. 2004;28:267–286. [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, Clinical and Experimental Research. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz SMM, Oscar-Berman M. Closing the gender gap: The case for gender-specific alcoholism research. Journal of Alcoholism and Drug Dependence. 2013;1:1–3. doi: 10.4172/2329-6488.1000e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues in Clinical Neuroscience. 2010;12:554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Monnot M, Vincent AS, Ross ED, Lovallo WR. Deficits in affective prosody comprehension: family history of alcoholism versus alcohol exposure. Alcohol and Alcoholism. 2010;45:25–29. doi: 10.1093/alcalc/agp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Bradizza CM, Gudleski GD, Coffey SF, Schlauch RC, Bailey ST, Bole CW, Gulliver SB. The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addictive Behaviors. 2012;37:469–476. doi: 10.1016/j.addbeh.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neuroscience and Biobehavioral Reviews. 2013;37:448–470. doi: 10.1016/j.neubiorev.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: A link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fouth Edition. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Fouth Edition. San Antonio, TX: The Psychological Corporation; 2009. [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wolwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: a MRI study. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]