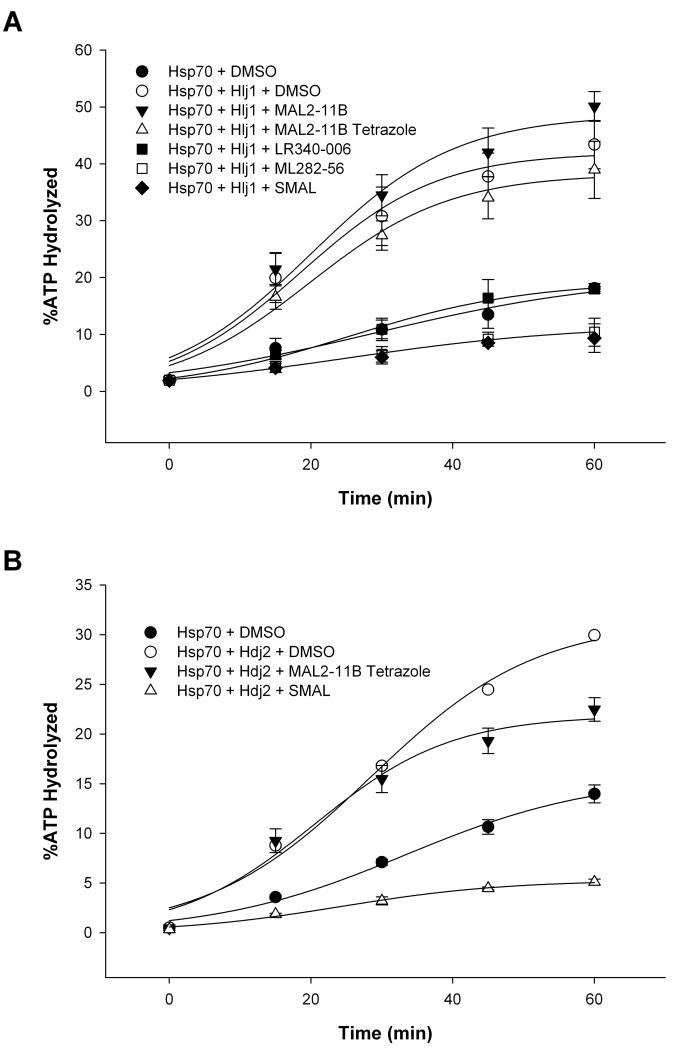
Figure 6.
Strategic modifications to the MAL2-11B tetrazole scaffold improve inhibition of J domain stimulation of Hsp70-mediated ATPase activity. Steady state ATPase assays were used to quantitate the percent ATP hydrolyzed by human Hsp70 over a 60-min time course in the presence of a J domain containing yeast protein (Hlj1) or a human J domain-containing protein (Hdj2) in parts (A) and (B), respectively, and in the presence of the indicated compounds at a final concentration of 100 μM versus a DMSO control. Data represent the means of three independent experiments, +/− SD, and were fitted using a sigmoidal, 3-parameter line regression. Purified Hlj1 and Hdj2 lacked significant levels of ATP hydrolysis (data not shown).