Figure 2.
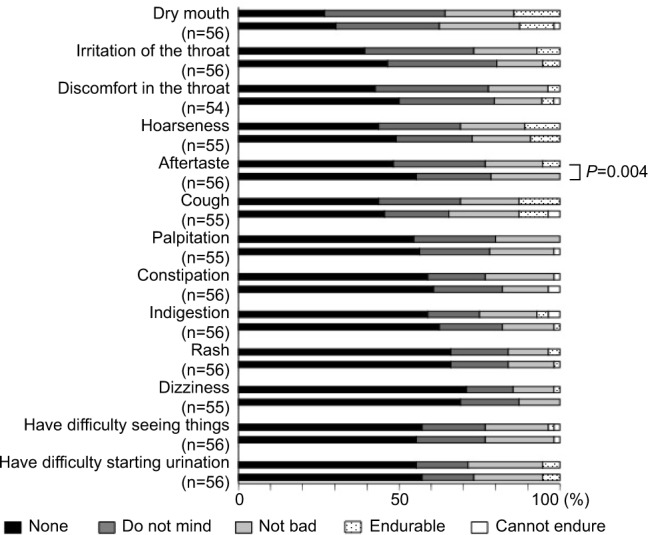
Questionnaire on adverse events 8 weeks after switching from the HandiHaler inhaler to the Respimat inhaler.
Notes: Respimat had a significantly milder aftertaste than the HandiHaler (P=0.004), but there were no other significant differences in the incidence of adverse events (question 4: Please indicate whether you experienced any of the following symptoms after using each device).
