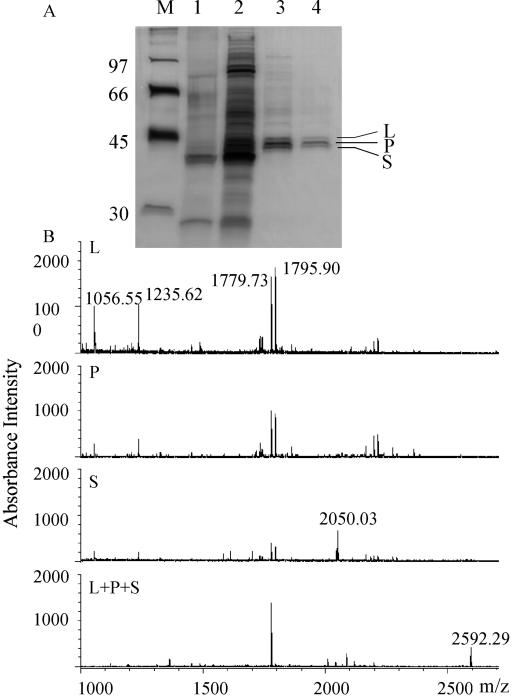
Figure 3.
A, SDS-PAGE of POMT-active fractions after successive steps of purification. The gel was silver stained. M, Molecular mass markers (numbers on the left indicate molecular mass in in kD); 1, Protein after G-25 Sephadex filtration (approximately 150 ng); 2, Protein after ammonium sulfate precipitation (approximately 1,500 ng); 3, Protein after first Poros HQ fractionation (approximately 150 ng); 4, Protein after second Poros HQ fractionation (approximately 75 ng). Three bands designated L (44 kD), P (43 kD), and S (42 kD) remain after the completed purification procedure. B, MALDI reflector mass spectra of tryptic peptides obtained from either of three gel-purified putative POMT proteins (L, P, and S) or from a mixture (L + P + S).