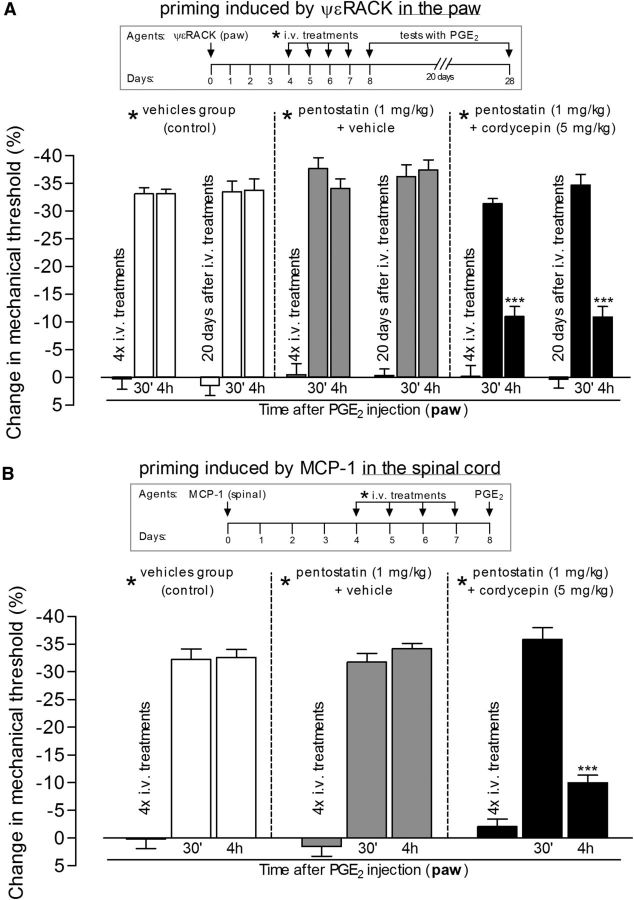
Figure 6.
Systemic treatment with protein translation inhibitor reverses hyperalgesic priming in both the paw and spinal cord. Hyperalgesic priming was induced by intradermal injection of the PKCε activator ψεRACK (1 μg) in the paw (A) or by spinal injection of MCP-1 (20 ng/μl, 20 μl; B). Four days later, as indicated by * in the schematics on the top of the figures, when the mechanical nociceptive paw withdrawal thresholds had returned to baseline values (data not shown), different groups of rats received intravenous vehicles (control groups, white bars), pentostatin (1 mg/kg) plus vehicle (gray bars), or pentostatin plus cordycepin (5 mg/kg, black bars), for 4 consecutive days. On the fifth day, PGE2 (100 ng) was injected into the dorsum of the hindpaw, and the mechanical nociceptive paw withdrawal thresholds were evaluated 30 min and 4 h later. There were no significant differences in the average paw withdrawal thresholds before the injection of PGE2 when compared with the thresholds before the injections of the priming stimuli (paired Student's t test): A: vehicle-plus-vehicle group, 111.6 ± 1.6 and 112.3 ± 1.7 g, respectively (t(5) = 0.2454; p = 0.8159, NS); pentostatin-plus-vehicle group, 108.3 ± 3.0 and 111.6 ± 0.9 g, respectively (t(5) = 1.300; p = 0.2504, NS); cordycepin-plus-pentostatin group, 120.0 ± 2.5 and 118.0 ± 2.0 g, respectively (t(5) = 0.7746; p = 0.4736, NS); B: vehicle-plus-vehicle group, 117.6 ± 2.6 and 114.3 ± 2.0 g, respectively (t(5) = 1.976; p = 0.1051, NS); pentostatin-plus-vehicle group, 111.6 ± 1.6 and 111.0 ± 1.3 g, respectively (t(5) = 0.5976; p = 0.5761, NS); cordycepin-plus-pentostatin group, 113.3 ± 2.1 and 112.0 ± 1.0 g, respectively (t(5) = 0.7906; p = 0.4650, NS). Of note, the systemic treatment with the vehicles, pentostatin, or cordycepin (or the combinations), after priming was induced (4 d after injection of ψεRACK or MCP-1) did not induce significant changes on the mechanical thresholds: A: vehicle-plus-vehicle group, t(5) = 1.118; p = 0.3144, NS; pentostatin-plus-vehicle group, t(5) = 0.4152; p = 0.6452, NS; cordycepin-plus-pentostatin group, t(5) = 0.1395; p = 0.8945, NS; B, vehicle-plus-vehicle group, t(5) = 1.400; p = 0.2204, NS; pentostatin-plus-vehicle group, t(5) = 2.739; p = 0.4090, NS; cordycepin-plus-pentostatin group, t(5) = 0.6523; p = 0.5430, NS. Two-way repeated-measures ANOVA followed by Bonferroni post-test showed that in the groups treated with cordycepin plus pentostatin, but not in the controls (vehicles or pentostatin plus vehicle), the hyperalgesia induced by PGE2 was significantly attenuated at the fourth hour [A: ***p < 0.001, when the PGE2-induced hyperalgesia at the 4 h time point in the cordycepin-plus-pentostatin group is compared with the control groups: cordycepin-plus-pentostatin × vehicles group: F(1,50) = 62.66, p < 0.0001; cordycepin-plus-pentostatin × pentostatin-plus-vehicle groups: F(1,50) = 58.22, p < 0.0001; nonsignificant (F(1,50) = 6.45, p > 0.05) difference was observed between vehicles × pentostatin-plus-vehicle groups; B: ***p < 0.001, when the PGE2-induced hyperalgesia at the 4 h time point in the cordycepin-plus-pentostatin is compared with the control groups: cordycepin-plus-pentostatin × vehicles group: F(1,20) = 16.30, p = 0.0024; cordycepin-plus-pentostatin × pentostatin-plus-vehicle groups: F(1,20) = 22.67, p = 0.0008; nonsignificant difference (F(1,20) = 0.01, p = 0.9394) was observed between vehicles × pentostatin-plus-vehicle groups], showing reversal of the ψεRACK- and MCP-1-induced hyperalgesic priming by systemic treatment with cordycepin. In addition, when tested for priming again by intradermal injection of PGE2 20 d later, the hyperalgesia at the fourth hour was still attenuated in the groups previously treated with cordycepin plus pentostatin (A, ***p < 0.001, when compared with the control groups), showing that the reversal of priming was very long lasting [N = 3 rats (6 paws) per group].