Figure 7.
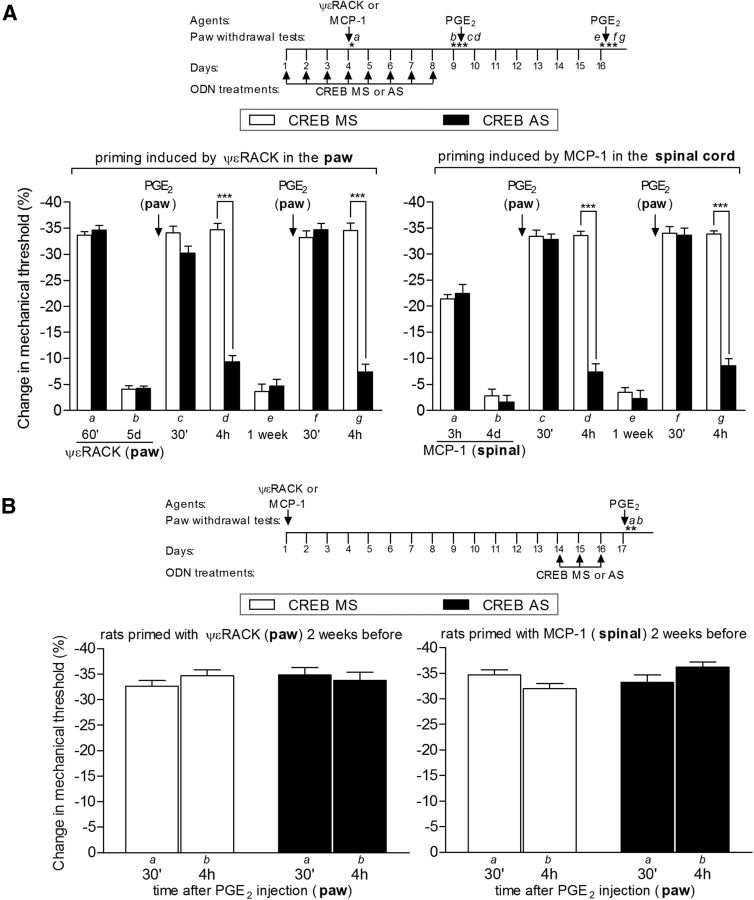
Knockdown of CREB prevents (A), but does not reverse (B), hyperalgesic priming. A, Rats were treated with daily spinal intrathecal injections of ODN AS (black bars) for CREB mRNA, for 3 consecutive days, to decrease its levels in the sensory neuron and prevent its activation by the priming inducers ψεRACK (1 μg, injected intradermally into the dorsum of the hindpaws; A, left) or MCP-1 (20 ng/μl; 20 μl, injected by the intrathecal route; A, right), injected on the fourth day. Control animals were treated, following the same protocol, with missense (MS; white bars). To prevent further activation of a signaling pathway that will ultimately produce priming during the acute effect of ψεRACK or MCP-1, the ODN treatments continued until the return of the mechanical nociceptive paw withdrawal thresholds to baseline values, when hyperalgesic priming was assessed by the administration of PGE2 (100 ng). PGE2 was injected intradermally into the dorsum of the hindpaw, and the mechanical threshold was evaluated 30 min and 4 h later. Average paw withdrawal thresholds before the injections of the priming stimuli and before the injection of PGE2 (8 d later) were as follows: ψεRACK in the paw, left, 121.3 ± 1.9 and 121.9 ± 1.4 g, respectively, for the CREB MS-treated group (t(11) = 0.000; p = 1.0000, NS), and 125.9 ± 1.7 and 125.5 ± 1.1 g, respectively, for the AS-treated group (t(11) = 0.1461; p = 0.8864, NS); MCP-1, intrathecally, right, 123.3 ± 2.5 and 120.5 ± 1.7 g, respectively, for the MS-treated group (t(11) = 1.787; p = 0.1014, NS) and 120.6 ± 2.3 and 116.5 ± 1.4 g, respectively, for the AS-treated group (t(11) = 1.948; p = 0.0773, NS). Paired Student's t test showed no significant difference between these two values. Two-way repeated-measures ANOVA followed by Bonferroni post-test showed significant mechanical hyperalgesia induced by PGE2 30 min after the injection. However, while in the MS-treated groups the magnitude of PGE2 hyperalgesia was still significant at the fourth hour, in the AS-treated groups it was strongly attenuated in both the ψεRACK- and the MCP-1-primed group (***p < 0.001 when the MS- and the AS-treated groups are compared). When tested again for priming with PGE2 1 week after the last treatment with ODN AS or MS, a time point when the levels of CREB had returned to pre-AS levels, the prolongation of PGE2-induced hyperalgesia was still attenuated (at the 4 h time point) in the ODN AS-treated groups, but not in the MS-treated groups, indicating a role of CREB in the induction of hyperalgesic priming [***p < 0.001 when the MS- and the AS-treated groups are compared; A, N = 6 rats (12 paws) per group]. Of note, no difference in the mechanical thresholds was observed at this time point, when compared with pre-priming stimuli thresholds: ψεRACK, left, t(11) = 0.8710; p = 0.4024 (NS) for the CREB MS-treated group, and t(11) = 1.497; p = 0.1624 (NS) for the AS-treated group; MCP-1, right, t(11) = 2.482; p = 0.305 (NS) for the MS-treated group, and t(11) = 1.915; p = 0.0819 (NS) for the AS-treated group (paired Student's t test). B, Rats that were treated with intradermal injection of the PKCε activator ψεRACK (1 μg) into the dorsum of the hindpaw (B, left) or spinal intrathecal injection of MCP-1 (20 ng/μl, 20 μl; B, right) 2 weeks before were treated with ODN AS (black bars) for CREB mRNA for 3 consecutive days to decrease the levels of CREB in the nociceptor. Control animals were treated, following the same protocol, with MS (white bars). On the fourth day, PGE2 (100 ng) was injected intradermally into the dorsum of the hindpaws, and the mechanical thresholds were evaluated 30 min and 4 h later. Average paw withdrawal thresholds before the injections of ψεRACK or MCP-1 and before the injection of PGE2 were as follows: ψεRACK, left, 121.6 ± 2.7 and 123.3 ± 1.6 g, respectively, for the CREB MS-treated group (t(11) = 0.8752; p = 0.4002, NS), and 113.3 ± 1.8 and 112.6 ± 2.1 g, respectively, for the AS-treated group (t(11) = 0.4838; p = 0.6380, NS); MCP-1, right, 114.1 ± 1.4 and 114.6 ± 1.2 g, respectively, for the MS-treated group (t(11) = 0.2536; p = 0.8045, NS) and 117.5 ± 1.7 and 117.5 ± 1.4 g, respectively, for the AS-treated group (t(11) = 0.0000; p = 1.0000, NS). Paired Student's t test showed no significant difference between these two values. Two-way repeated-measures ANOVA followed by Bonferroni post-test showed no difference in the magnitude of the PGE2-induced hyperalgesia at 30 min and 4 h in both the ODN AS- and MS-treated groups (rats primed with ψεRACK: F(1,22) = 1.47, p = 0.2378; rats primed with MCP-1: F(1,22) = 1.39, p = 0.2511), indicating that CREB does not play a role in the maintenance of hyperalgesic priming [B: N = 6 rats (12 paws) per group].