Figure 3. Deletion or a single point mutation in the ATP binding site of the TRPM7 kinase domain does not affect aldosterone-induced generation of reactive oxygen species (ROS) and NADPH oxidase activation.
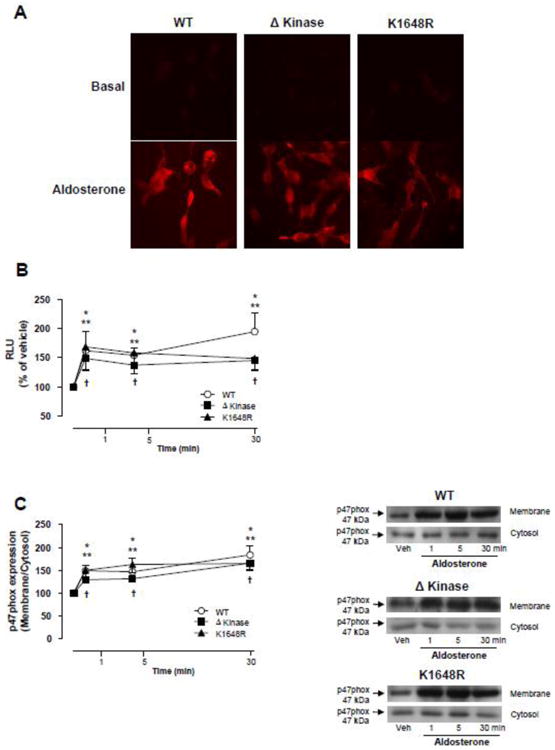
HEK-293 cells expressing WT hTRPM7 channels or the mutants ΔKinase (deleted kinase) and K1648R (inactive kinase) were stimulated with 100 nM aldosterone (1 to 30 min). (A) Representative images of intracellular DHE fluorescence in aldosterone stimulated cells (30 min). (B) Line graph, NADPH-derived ROS was measured by lucigenin chemiluminescence in homogenates from aldosterone-stimulated cells. The values were normalized by protein concentration in each sample and expressed as relative luminescence units (RLU). (C) NADPH oxidase p47phox cytosolic subunit translocates to the cell membrane upon aldosterone stimulation. Cell membrane and cytosol were fractionated and immunoblotted using antibody to p47phox. Line graph, effect of aldosterone on p47phox translocation from the cytosol to the cell membrane. Translocation was determined by the protein expression ratio in membrane to cytosol fractions. Side panels, representative immunoblots of p47 phox. Results are means ± S.E. (% of vehicle) of 7-10 independent experiments. *p<0.05 WT hTRPM7 versus vehicle; **p<0.05 ΔKinase versus vehicle;†p<0.05 K1648R versus vehicle.
