Abstract
Thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), and scleroderma renal crisis (SRC) all present with features of thrombotic microangiopathy. Distinguishing among these entities is critical, however, as treatments differ and may be mutually exclusive. We describe the case of a 25-year-old woman with an undefined mixed connective tissue disease who presented 6 weeks post-partum with fever, transient aphasia, thrombocytopenia, hemolytic anemia, and acute kidney injury eventually requiring initiation of hemodialysis. Renal biopsy revealed thrombotic microangiopathy. Renal function did not improve despite immediate initiation of plasma exchange, and an angiotensin-converting enzyme (ACE) inhibitor was initiated following discontinuation of plasma exchange. At last follow up, she remained dialysis dependent. Due to the myriad causes of thrombotic microangiopathy and potential for diagnostic uncertainty, the patient’s response to therapy should be closely monitored and used to guide modification of therapy.
Keywords: thrombotic microangiopathy, scleroderma, postpartum, mixed connective tissue disease
Introduction
Thrombotic microangiopathies are a diverse group of disorders that clinically present in a similar manner. Thrombotic thrombocytopenic purpura (TTP), first described in 1924, is a rare but potentially lethal condition [1]. The diagnosis is made clinically and is classically characterized by a pentad of thrombocytopenia, microangiopathic hemolytic anemia, transient neurological symptoms, renal dysfunction, and fever. Although the introduction of therapeutic plasma exchange in the 1970’s has improved survival rates, mortality remains 10 – 20% [2]. In 1998, the presence of large von Willebrand factor multimers in idiopathic TTP was ascribed to deficiency of the von Willebrand factor-cleaving protease (ADAMTS13) enzyme [3]. Hemolytic uremic syndrome (HUS) is most often associated with infection by Escherichia coli 0157:H7, which produces a verotoxin that mediates endothelial damage. Increasingly, atypical forms of HUS (aHUS) are recognized to result from disorders of complement regulation, including antibodies to or mutations in the genes encoding complement factors B, H, and I as well as cell surface marker CD46, complement component 3 (C3), and thrombomodulin [4]. Pathological specimens from target organs (kidneys, cerebral vasculature, and skin) reveal thrombotic microangiopathy (TMA). This finding is seen in a number of processes, including malignant hypertension, tumor cell embolism, paroxysmal nocturnal hemoglobinuria, humoral rejection in transplanted organs, antiphospholipid antibody syndrome, postpartum state, drug-mediated endothelial damage, and scleroderma renal crisis (SRC) [5]. SRC results in new onset of significant systemic hypertension and renal dysfunction [6]. Early treatment with angiotensin-converting enzyme (ACE) inhibitors has reduced 12-month mortality of SRC to less than 15% [7]. Distinguishing other causes of TMA from SRC can be difficult. However, simultaneous therapy with plasma exchange and an ACE inhibitor is contraindicated due to the potential for developing a bradykinin-mediated reaction resulting in severe hypotension [8].Existing literature discussing the diagnostic difficulty in differentiating other causes of TMA, such as post-partum TTP, from SRC is scarce [9]. Herein, we report the 6th case of scleroderma renal crisis (with overlap features of TTP) in mixed connective tissue disease (MCTD) [10, 11, 12, 13, 14], but only the second in the postpartum period.
Case report
A 25-year-old woman with mixed connective tissue disease (MCTD) presented to another institution 6 weeks post-partum with low-grade fever, generalized malaise, nausea, and dyspnea on exertion. Two transient episodes of aphasia with bilateral lower facial weakness were also reported. She had recently been restarted on prednisone 40 mg daily after developing dyspnea and pleuritis, thought to be an exacerbation of MCTD, which was characterized by positive antinuclear antibodies, anti-Smith antibody, and anti-ribonucleoprotein antibody. Anti-topoisomerase antibodies (anti-Scl-70) and anti-centromere antibodies were negative. Upon presentation, she had uncontrolled hypertension, periorbital edema, and livedo reticularis. On admission to our institution, she was noted to have non-oliguric acute kidney injury with serum creatinine 4.3 mg/dl, nephrotic range proteinuria, normocytic anemia with hemoglobin 10.4 g/dl, and thrombocytopenia with a platelet count of 63 × 109/l. As illustrated in Figure 1, the anemia and thrombocytopenia were progressive, and lactate dehydrogenase (LDH) was elevated. Haptoglobin level was low at 23 mg/dl. Complement levels were normal. Rare schistocytes were seen on peripheral blood smear, consistent with hemolysis. A renal biopsy was performed, revealing active and chronic thrombotic microangiopathy (Figure 2) by light microscopy processed urgently. In view of recent neurologic symptoms, acute kidney injury, thrombocytopenia, microangiopathic hemolytic anemia, and history of low grade fever, a presumptive diagnosis of TTP was made. Plasma exchange was initiated emergently, and prednisone was increased to 80 mg daily with subsequent improvement in platelet count and LDH. In addition, hemodialysis was initiated for worsening kidney injury.
Figure 1. Laboratory data during clinical course.
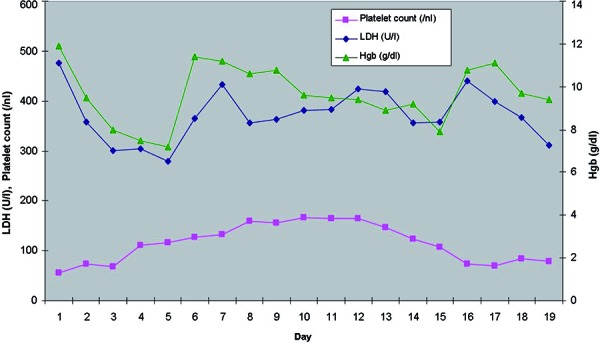
Figure 2. Masson’s trichrome stain (× 40) showing glomerulus with segmental thrombosis and mesangiolysis.
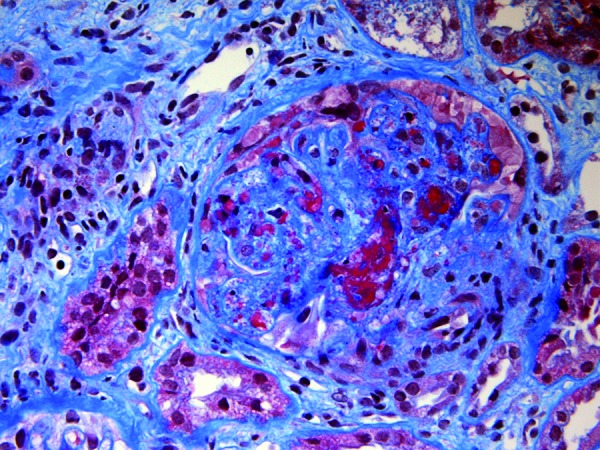
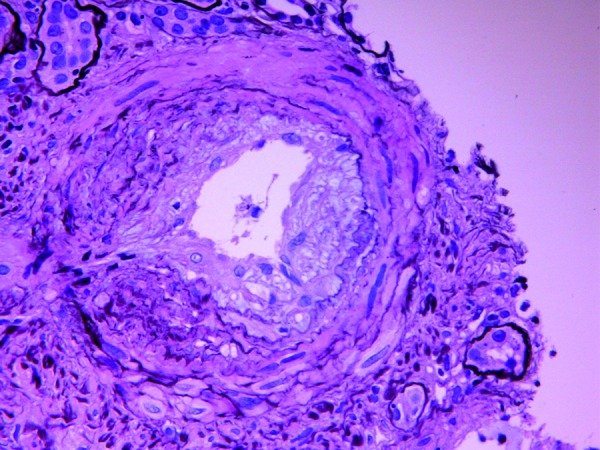
Figure 3. Jones’ methenamine silver stain (× 40) showing renal artery with prominent myxoid intimal thickening.
After four sessions of plasma exchange, however, LDH began to rise despite normalization of haptoglobin and resolution of schistocytes on peripheral smear (Figure 1). The ADAMTS13 level, sent prior to initiation of plasma exchange, returned as normal. As the patient’s course evolved, the concern was raised for a possible scleroderma process rather than a manifestation of chronic TTP. Plasma exchange was discontinued, and the patient was initiated on captopril for presumed scleroderma renal crisis. Her previous antihypertensive regimen had consisted of amlodipine, metoprolol, and clonidine. While the microangiopathic hemolytic anemia and thrombocytopenia resolved, renal function did not improve, and she remained dialysis dependent 5 months after the initial diagnosis.
Discussion
This case illustrates the diagnostic difficulty in differentiating among the various causes of TMA in a post-partum female with a history of MCTD. Table 1 compares clinical characteristics of various causes of peripartum TMA. Castella et al. reported that among patients with TTP, 10 – 25% were pregnant or in the postpartum period [15, 16]. The pentad classically seen with TTP, which results from microvascular platelet clumping [9], can also arise with SRC. Prompt initiation of therapy (plasmapheresis) for the former is mutually exclusive with that (ACE inhibition) of the latter given the threat of a bradykinin-mediated reaction [8]. Several strategies may be used to distinguish between the two conditions. First, while the pathophysiology of TTP is typically mediated by deficiency of von Willebrand factor cleaving protease (ADAMTS13), SRC results from endothelial injury and intimal proliferation [17]. Although ADAMTS13 activity has not been studied in SRC, the assay has good specificity for “idiopathic” TTP (91% in one large case series) [18]. Consequently, severe ADAMTS 13 deficiency (< 5%) may be used to help confirm the clinical diagnosis of idiopathic TTP. High suspicion for TTP typically mandates rapid initiation of treatment without the luxury of an ADAMTS13 level given the prolonged turnaround time, but the assay can be incorporated later in the clinical course. While considered by some to be entities along the same spectrum as TTP, some forms of aHUS are now known to be due to dysregulation at various points in the complement system [4] with testing for these disorders not widely available clinically. This further complicates arrival at an accurate and timely diagnosis of a patient presenting with TMA.
Table 1. Comparison of common clinical characteristics of causes of peripartum TMA [17, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34].
| TTP | “Typical” HUS | Atypical HUS | Pre-eclampsia | HELLP | SRC | APS | |
|---|---|---|---|---|---|---|---|
| Clinical presentation | Fever, HTN, neurologic symptoms, bleeding, purpuric rash | Abdominal pain, bloody diarrhea due to verotoxin-production; neurologic symptoms possible | None or non-specific prodrome with malaise, fatigue, upper respiratory symptoms | HTN, nausea, vomiting, abnormal vision | Abdominal pain, headache, malaise, nausea, vomiting, HTN | Dyspnea, altered mentation, HTN | HTN, arterial and venous thromboses, fetal demise |
| Typical laboratory findings | MAHA, ↓platelets, AKI, proteinuria, hematuria |
MAHA, ↓platelets, AKI, proteinuria, hematuria |
MAHA, ↓platelets, AKI, proteinuria, hematuria, ↓complement |
Proteinuria, hyperuricemia |
MAHA, ↑AST, ↓platelets |
MAHA, AKI, proteinuria, hematuria |
MAHA, ↓platelets, AKI, proteinuria, hematuria, antiphospholipid antibodies |
| Other possible laboratory findings | Low level of ADAMTS13 | Stool culture positive for verotoxin-producing E. coli | Abnormalities of complement regulatory proteins (Factors B, H, and I, MCP, C3, thrombomodulin) | MAHA, AKI, and ↑AST may occur in severe pre-eclampsia | AKI | Autoantibodies suggestive of scleroderma | Autoantibodies suggestive of SLE |
| Occurrence and timing related to pregnancy |
Rare; generally < 23 – 26 weeks gestation | Rare; post-partum | Rare; post-partum | > 20 weeks gestation; occasionally postpartum | > 20 weeks gestation; occasionally postpartum | Unclear; > 24 weeks gestation when observed | 1/3 of cases reported during pregnancy or postpartum period |
| Treatment | Plasma exchange, steroids, rituximab | Supportive | Plasma exchange, eculizumab | Anti-HTN therapy; when severe, magnesium sulfate and delivery | Delivery | ACE-inhibitor therapy | Anticoagulation, plasma exchange |
| Renal prognosis | ESRD is rare (0 – 6%) | CKD in 5 – 25% | ESRD in 20 – 60% | Low risk of ESRD (~ 8%) | ESRD is rare (0 – 2%) | ESRD in 20% treated with ACE-inhibitor | ESRD is rare (few case reports) |
TMA = thrombotic microangiopathy; TTP = thrombotic thrombocytopenic purpura; HUS = hemolytic uremic syndrome; SRC = scleroderma renal crisis; APS = antiphospholipid antibody syndrome; HELLP = hemolysis, elevated liver enzymes, low platelets; HTN = hypertension; MAHA = microangiopathic hemolytic anemia; AKI = acute kidney injury; AST = aspartate aminotransferase; ADAMTS = a disintegrin-like and metalloprotease with thrombospondin type 1 motif; MCP = membrane cofactor protein; SLE = systemic lupus erythematosus; ACE = angiotensin converting enzyme; ESRD = end-stage renal disease; CKD = chronic kidney disease.
Second, the diagnosis of SRC needs to be strongly considered in patients with systemic sclerosis since 25% of that subset of patients develops SRC compared to 1% of those with the limited cutaneous form of scleroderma [19]. While our patient demonstrated no cutaneous evidence of scleroderma, including a skin biopsy, systemic sclerosis sine scleroderma was a consideration. This is an entity associated with undifferentiated connective tissue disease in which organ involvement of scleroderma is witnessed without skin involvement [20]. In these patients, SRC with poor, and even fatal, outcomes can occur [10, 15, 21]. Although it is well-established that connective tissue disorders may be exacerbated by pregnancy, it is unclear if rates of SRC are increased in pregnant women [22]. Another consideration in this case is the use of high-dose corticosteroids, which are associated with SRC [23]. While it is reasonable to implicate pregnancy as a potential precipitant given the timing of presentation, steroid exposure may have been an important risk factor. Additionally, worsening renal function was observed prior to increase of steroid dose.
Finally, response to treatment must be critically monitored. The only established treatment of SRC is ACE inhibitor therapy [24] while early initiation of plasma exchange in other causes of TMA is often lifesaving [9]. Due to the possibility of developing severe hypotension, administration of ACE inhibitors is contraindicated in patients undergoing plasma exchange. It is recommended that ACE inhibitor therapy be discontinued 24 – 72 hours prior to initiation of plasma exchange, depending on the duration of action of the ACE inhibitor [8]. Failure of plasma exchange for suspected TTP or aHUS should prompt reconsideration of alternate diagnoses, such as SRC, and the treatment plan should be adjusted as indicated.
References
- 1. Verbeke L Delforge M Dierickx D Current insight into thrombotic thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2010; 21: 3–10. [DOI] [PubMed] [Google Scholar]
- 2. Kiss JE Thrombotic thrombocytopenic purpura: recognition and management. Int J Hematol. 2010; 91: 36–45. [DOI] [PubMed] [Google Scholar]
- 3. Furlan M Robles R Galbusera M Remuzzi G Kyrle PA Brenner B Krause M Scharrer I Aumann V Mittler U Solenthaler M Lämmle B von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998; 339: 1578–1584. [DOI] [PubMed] [Google Scholar]
- 4. Rosales A Riedl M Zimmerhackl LB Thrombotic microangiopathy: atypical HUS: current diagnostic and therapeutic approaches. Nat Rev Nephrol. 2010; 6: 504–506. [DOI] [PubMed] [Google Scholar]
- 5. Benz K Amann K Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens. 2010; 19: 242–247. [DOI] [PubMed] [Google Scholar]
- 6. Denton CP Lapadula G Mouthon L Müller-Ladner U Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009; 48: iii32–iii35. [DOI] [PubMed] [Google Scholar]
- 7. Bussone G Bérezné A Pestre V Guillevin L Mouthon L The scleroderma kidney: progress in risk factors, therapy, and prevention. Curr Rheumatol Rep. 2011; 13: 37–43. [DOI] [PubMed] [Google Scholar]
- 8. Weinstein R Hypocalcemic toxicity and atypical reactions in therapeutic plasma exchange. J Clin Apher. 2001; 16: 210–211. [DOI] [PubMed] [Google Scholar]
- 9. Manadan AM Harris C Block JA Thrombotic thrombocytopenic purpura in the setting of systemic sclerosis. Semin Arthritis Rheum. 2005; 34: 683–688. [DOI] [PubMed] [Google Scholar]
- 10. Andersen GN Vasko J Scleroderma renal crisis and concurrent isolated pulmonary hypertension in mixed connective tissue disease and overlap syndrome: report of two cases. Clin Rheumatol. 2002; 21: 164–169. [DOI] [PubMed] [Google Scholar]
- 11. Celikbilek M Elsurer R Afsar B Ozdemir HB Sezer S Ozdemir NF Mixed connective tissue disease: a case with scleroderma renal crisis following abortion. Clin Rheumatol. 2007; 26: 1545–1547. [DOI] [PubMed] [Google Scholar]
- 12. Crapper RM Dowling JP Mackay IR Whitworth JA Acute scleroderma in stable mixed connective tissue disease: treatment by plasmapheresis. Aust N Z J Med. 1987; 17: 327–329. [DOI] [PubMed] [Google Scholar]
- 13. Greenberg SA Amato AA Inflammatory myopathy associated with mixed connective tissue disease and scleroderma renal crisis. Muscle Nerve. 2001; 24: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 14. Satoh K Imai H Yasuda T Wakui H Miura AB Nakamoto Y Sclerodermatous renal crisis in a patient with mixed connective tissue disease. Am J Kidney Dis. 1994; 24: 215–218. [DOI] [PubMed] [Google Scholar]
- 15. Kanso AAHN Badr KF Microvascular and macrovascular diseases of the kidney In: Brenner BM(ed). Brenner and Rector’s The Kidney 8th Edition. Philadelphia, PA: Saunders Elsevier; 2007, chap 32. [Google Scholar]
- 16. Castellá M Pujol M Juliá A Massague I Bueno J Ramón Grifols J Puig L Thrombotic thrombocytopenic purpura and pregnancy: a review of ten cases. Vox Sang. 2004; 87: 287–290. [DOI] [PubMed] [Google Scholar]
- 17. D’Angelo A Fattorini A Crippa L Thrombotic microangiopathy in pregnancy. Thromb Res. 2009; 123: S56–S62. [DOI] [PubMed] [Google Scholar]
- 18. Just S Methodologies and clinical utility of ADAMTS-13 activity testing. Semin Thromb Hemost. 2010; 36: 82–90. [DOI] [PubMed] [Google Scholar]
- 19. Steen VD Scleroderma renal crisis. Rheum Dis Clin North Am. 2003; 29: 315–333. [DOI] [PubMed] [Google Scholar]
- 20. Slobodin G Rosner I Rozenbaum M Naschitz JE Zuckerman E Yeshurun D Systemic sclerosis sine scleroderma: is it always the same disease? Report of three patients and discussion. Rheumatol Int. 2002; 22: 170–172. [DOI] [PubMed] [Google Scholar]
- 21. Szigeti N Fábián G Czirják L Fatal scleroderma renal crisis caused by gastrointestinal bleeding in a patient with scleroderma, Sjögren’s syndrome and primary biliary cirrhosis overlap. J Eur Acad Dermatol Venereol. 2002; 16: 276–279. [DOI] [PubMed] [Google Scholar]
- 22. Chakravarty EF Vascular complications of systemic sclerosis during pregnancy. Int J Rheumatol. 2010; 5 pages [DOI] [PMC free article] [PubMed]
- 23. Trang G et al. Corticosteroids and the risk of scleroderma renal crisis: a systematic review. Rheumatology international 2010; [DOI] [PubMed]
- 24. Steen VD Costantino JP Shapiro AP Medsger TA Outcome of renal crisis in systemic sclerosis: relation to availability of angiotensin converting enzyme (ACE) inhibitors. Ann Intern Med. 1990; 113: 352–357. [DOI] [PubMed] [Google Scholar]
- 25. Hagel S Jantsch J Budde U Kalden JR Eckardt KU Veelken R Treatment of acquired thrombotic thrombocytopenic purpura (TTP) with plasma infusion plus rituximab. Thromb Haemost. 2008; 100: 151–153. [DOI] [PubMed] [Google Scholar]
- 26. Gabbe SGNJ Simpson JL ed. Obstetrics: Normal and Problem Pregnancies. Philadelphia, PA: Elsevier; 5th ed. 2005; [Google Scholar]
- 27. Fakhouri F Frémeaux-Bacchi V Does hemolytic uremic syndrome differ from thrombotic thrombocytopenic purpura? Nat Clin Pract Nephrol. 2007; 3: 679–687. [DOI] [PubMed] [Google Scholar]
- 28. Espinosa G Bucciarelli S Cervera R Lozano M Reverter JC de la Red G Gil V Ingelmo M Font J Asherson RA Thrombotic microangiopathic haemolytic anaemia and antiphospholipid antibodies. Ann Rheum Dis. 2004; 63: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steen VD Medsger TA Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000; 133: 600–603. [DOI] [PubMed] [Google Scholar]
- 30. Uthman I Khamashta M Antiphospholipid syndrome and the kidneys. Semin Arthritis Rheum. 2006; 35: 360–367. [DOI] [PubMed] [Google Scholar]
- 31. Amirlak I Amirlak B Haemolytic uraemic syndrome: an overview. Nephrology (Carlton). 2006; 11: 213–218. [DOI] [PubMed] [Google Scholar]
- 32. Conlon PJ et al. The renal manifestations and outcome of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in adults. Nephro Dial Transplant. 1995; 10: 1189-1193. [PubMed] [Google Scholar]
- 33. Vikse BE Irgens LM Leivestad T Skjaerven R Iversen BM Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008; 359: 800–809. [DOI] [PubMed] [Google Scholar]
- 34. Habli M et al. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol. 2009; 201 385: e1-5. [DOI] [PubMed] [Google Scholar]


