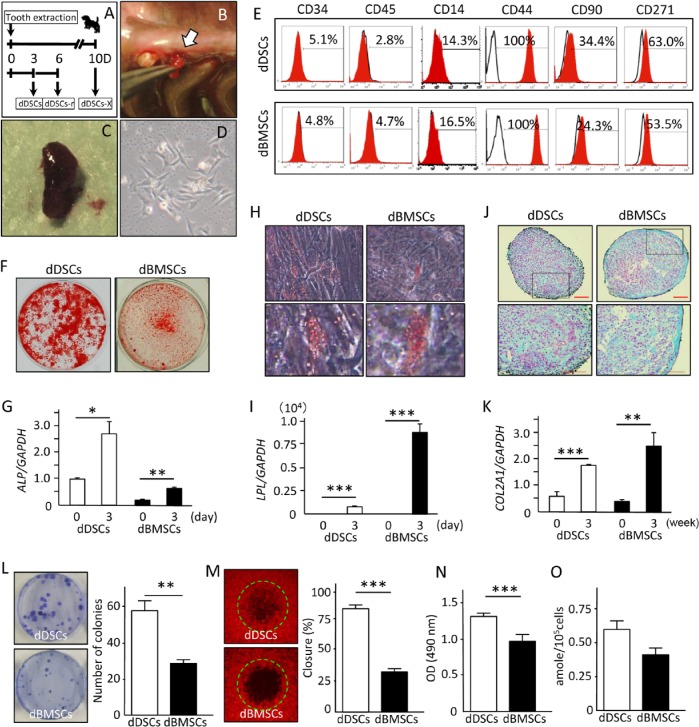
Figure 2.
Isolation and characterization of the dog mesenchymal stem/progenitor cells. (A) Schematic illustration of the experimental design for collecting dental socket–derived cells (DSCs). (B, C) Photograph of the granulation tissue collected from the dental socket 3 d after tooth extraction. (D) DSC morphology at the first passage. (E) Cell surface characterization of dog DSCs (dDSCs) and dog bone marrow–derived mesenchymal stem/progenitor cells (dBMSCs) with flow cytometric analysis. (F-K) Mouse dental socket–derived cells (mDSCs) and dBMSCs were cultured in specific induction medium for 21 d to induce cell differentiation into osteo-, adipo-, and chondrogenic lineages and then stained with alizarin red S (F), oil red O (H), or alcian blue (J). Expression levels of the differentiation marker genes ALP (G), LPL (I), and COL2A1 (K) for osteo-, adipo-, and chondrogenesis, respectively, were evaluated by real-time reverse transcription polymerase chain reaction analysis. Four independent experiments with at least 3 wells were performed with cells obtained from 3 dogs. Scale bar (J), 100 µm. (L) Images and quantitative results of the CFU-F assay showing a significantly higher number of colonies in the dDSCs. Two independent experiments were performed in quadruplicate, with cells from 2 dogs. (M) The degree of cell motility was evaluated with the OrisCell Migration Assay kit 6 hr after cell plating. Images of each well were captured with fluorescence microscopy, and the closing ratio was measured with the NIH image software program. Experiments were performed in quadruplicate, with cells from 2 dogs. (N) MTS assay showing a significantly higher proliferation ability of dDSCs. Data representative of 2 independent experiments with 6 wells per experiment, from cells of 2 dogs. (O) Telomerase assay showing a higher but not statistically significant activity in dDSCs than in dBMSCs. Data are representative of 3 independent experiments performed with cells from 3 dogs. * p < .05. ** p < .01. *** p < .001. t test.