Abstract
Zirconium dispersed in aluminum-pillared montmorillonite was prepared as a catalyst for phenol hydroxylation. The effects of varying the Zr content on the catalyst’s physicochemical character and activity were studied with XRD, BET surface area analysis, surface acidity measurements and scanning electron microscopy before investigating the performance for phenol conversion. The zirconia dispersion significantly affects the specific surface area, the total surface acidity and surface acidity distribution related to the formation of porous zirconia particles on the surface. The prepared samples exhibited excellent catalytic activity during phenol hydroxylation.
Keywords: Pillared clay, Phenol, Hydroxylation, Zirconia, Catalysis
Introduction
Smectite and pillared smectite clays have attracted increasing interest due to their application as heterogeneous catalysts in both gas and liquid systems; their innate surface acidity might play an important role in many reactions. The silicate interlayer in the clay’s structure accommodates the adsorption–desorption mechanism of the reactants and products in heterogeneous surface mechanisms; however, the layers undergo collapse easily at high temperatures, limiting these materials’ application as catalysts in high-temperature reactions. Pillared smectite was engineered to improve the catalytic activity by increasing mass transport of reactants and products, as well as to increase the catalyst’s thermal stability. Because the changes in the pore distribution tend to form interlayer pores during pillarization, pillared smectite can be applied in numerous purposes, including acting as a molecular sieve and a heterogeneous catalyst. The synthetic method utilized to manufacture this material and its applications have also been heavily studied [1,2]. In addition, transition metal pillared smectite catalyst provides specific surface active sites for several reactions, including some redox processes. Pore formation and distribution in the metal oxide pillared clays lend its own advantages relative to the increased transport of reactants and products and selectivity toward the appropriate product in the catalytic mechanism [3–9].
Pillaring smectite with aluminum oxide is a pillarization technique that uses some widely studied and reported metal oxides. Aluminum-pillared smectite was discovered during previous studies, revealing an enhancement in the thermal stability and the possibility that it may used as a support [10–12]. One interesting approach utilizes aluminum-pillared smectite to support dispersed ZrO2 and ZrO2-pillared clay. The catalytic properties related to the potential surface acidity gained from catalyst’s form can accelerate, forming peroxide radicals during the hydroxylation reaction, as well as the photo-Fenton reaction and alkylation reactions. Hypothetically, the superior nature of the pillared smectite clays during its use as a catalyst support can accommodate the effectiveness of the active site catalysis while increasing the homogeneous dispersion and stability of the catalyst.
Studies in the literature related to zirconia-pillared clay and the combination of zirconia with aluminum-pillared montmorillonite are limited, although ZrO2 is a well-known active metal oxide catalyst utilized in many chemical reactions. Previous investigations have revealed that ZrO2 pillarization significantly affected the montmorillonite’s catalytic activity in some organic reactions, including the linear alkylation of benzene, acylation of diol, and acetylation, while ZrO2 dispersed into aluminum-pillared saponite has been used for NOx selective catalytic reduction (SCR) and phenol hydroxylation [13–16]. Al-pillared montmorillonite enhances the catalytic activity of ZrO2, while the ZrO2 increases the catalyst stability. From a previous study regarding the combination of ZrO2 and aluminum-pillared montmorillonite, higher reaction rates were obtained and it was shown that the advanced hydroxylation of phenol for producing additional products of catechol (CAT) and benzoquinone (BQ) is an important characteristic of ZrO2/Al-MMT as a catalyst [17]. Based on previous investigations suggesting that the catalyst preparation was influenced by the preparation parameters, this study focused on the effects of Zr’s cation exchange capacity (CEC) ratio relative to its physicochemical characteristics and its activity as a catalyst in phenol hydroxylation.
Experimental
Materials
Naturally occurring montmorillonite from PT. Tunas Inti Makmur, Semarang, Indonesia, was received in powder form and used as the main material for preparation. To minimize the amount of impurities, before the pillarization process, the montmorillonite was activated by refluxing in sulfuric acid for 6 h and neutralized by washing with distilled water until the solid was free of Cl− and the filtrate had a pH equal to 7. Other chemicals, including zirconium isopropoxide, phenol, methanol, benzoquinone and hydrogen peroxide were obtained from E. Merck.
Preparation of ZrO2/Al-MMT
ZrO2/Al-MMT was prepared in two major steps: aluminum pillarization and ZrO2 dispersion into the aluminum-pillared montmorillonite. The aluminum pillarization of montmorillonite clay was conducted by preparing a Keggin ion precursor; this substance was obtained by slowly titrating an aluminum chloride solution with NaOH under vigorous stirring until a 2.2 −OH/Al ratio was attained. The Keggin ion solution was added dropwise to a suspension of activated montmorillonite to achieve a 10 mmol/gAl content. The suspension was filtered and neutralized before being dried and calcined at 500 °C for 6 h. Through these steps, aluminum-pillared montmorillonite was obtained and encoded as Al-MMT.
The precursor solution used for ZrO2 dispersion was prepared by mixing zirconium isopropoxide into isopropanol with stirring for 4 h. The resultant clear solution was then added into the Al-MMT powder at a certain ratios of Zr relative to the cation exchange capacity (CEC) (Zr/CEC) of Al-MMT. In an earlier investigation, the CEC of the Al-MMT was 45 meq/100 g. The Zr/CEC ratio was varied at 10, 20, 40 and 60. The mixture containing the Zr precursor and the Al-MMT was stirred for 24 h at room temperature before it was filtered, dried and calcined. The calcination was performed by heating the solids at 400 °C for 4 h. According to the varied Zr content, the ZrO2/Al-MMT materials were encoded as 10Zr, 20Zr, 40Zr or 60Zr, with the number indicating the Zr to CEC ratio in dispersion.
Material characterization
The physicochemical characteristics of the prepared materials were determined using conducted several instrumental analysis, including X-ray diffraction, BET surface area analysis and scanning electron microscope-energy dispersive X-ray (SEM-EDX). A NOVA 1200e Gas Sorption Analyzer and a SEM-EDX by Seiko were used for these analyses. The XRD patterns of these materials were obtained by packing finely ground samples into an aluminum sample holder and scanning from 2° to 60° of 2θ at 2°/min. A Shimadzu X6000 diffractometer with a Ni-filtered source was used. The interlayer distance (d001) was calculated using the measured diffraction angle (2θ) with the Bragg equation. The surface acidity of the materials was determined using the total solid acidity measured as the mmole/gram of butylamine adsorbed by solid sample that was determined using a back titration method and surface distribution by pyridine-adsorption followed by Fourier-Transform infra-red (FTIR) analysis with a Nicolet Avatar Spectrophotometer. To measure the total acidity, solid samples were treated with 0.1 N butylamine in acetonitrile for 24 h. The butylamine adsorbed by the solid represents the amount of acid sites on the solid’s surface and was calculated by titration to the unreacted n-butylamine. For a different methods, the acid distribution in the solid samples was studied by adsorbing pyridine onto the solid samples, followed by identification by FTIR. Lewis and Brønsted acidic species can be detected by comparing the spectral intensity. These types of sites that affect adsorption were classified as Lewis (LPY), Brønsted (BPY) and surface hydroxyl (HPY) sites [17,18].
The ratio of the Brønsted to the Lewis was calculated based on the ratio between the absorption at 1560 cm−1 and the absorption spectrum at 1450 cm−1 [19,20].
The catalytic hydroxylation of phenol was carried out in a stirred (stirring rates of between 500 and 1000 rpm) and thermostated Pyrex well-mixed slurry batch reactor of 250 mL. The reaction was performed at reflux: a 10 ppm phenol solution must watch in aqueous solution in contact with varied Zr/Al-MMT catalysts under continuous stirring. After 5 min of stirring, a hydrogen peroxide solution was added in a 1/1 molar ratio of phenol to H2O2, and this addition was measured as time zero of the reaction. The analysis of the products during the sampling time was undertaken by collecting samples (5 μL) and analyzing them using high performance liquid chromatography (HPLC). For quantitative determinations, an external standard (phenol and hydroquinone solution) was used during HPLC analyses and combined with gas chromatography–mass spectrometry (GC/MS) analyses.
Results and discussion
The elemental analysis data for the materials are listed in Table 1.
Table 1.
Elemental analysis of prepared material, MMT and Al-MMT.
| Sample | Component (wt.%) |
||||
|---|---|---|---|---|---|
| Na2O | MgO | SiO2 | Al2O3 | ZrO2 | |
| MMT | 8.33 | 2.82 | 59.80 | 27.33 | nd |
| Al-MMT | 5.21 | 1.38 | 51.52 | 38.2 | nd |
| 10Zr | 2.21 | 0.32 | 50.45 | 37.88 | 1.92 |
| 20Zr | 1.19 | 0.31 | 49.57 | 36.05 | 2.21 |
| 40Zr | 1.17 | 0.38 | 48.06 | 32.32 | 3.09 |
| 60Zr | 1.14 | 0.39 | 47.89 | 35.99 | 7.16 |
For all materials, Al2O3 and SiO2 are the dominant components, as indicated by the main structure of montmorillonite. After it was pillared by Al2O3, the content of Al increased. The Zr content values of the materials showed a linear relationship between Zr loading in the impregnation procedure and the content of Zr in the obtained materials. Furthermore, the effect of the Zr content on the structure of the material was characterized by XRD analysis. The XRD patterns of the materials are depicted in Fig. 1.
Fig. 1.

From bellow to above: XRD pattern of Al-MMT, 10Zr, 20Zr, 40Zr and 60Zr.
The XRD patterns for the montmorillonite exhibited the characteristic reflections at 2θ = 6.3° (d001 = 14.9 Å) and 2 = 19.9° (d = 4.5 Å). The other reflections at 21.8° and 26.6° were attributed to crystobalite and quartz. After pillarization by Al2O3, the [0 0 1] reflection was shifted to the lower angle, which corresponded to the d001 increase from 14.58 Å to 16.22 Å due to the successful pillarization. The presence of zirconia at the surface results from the formation of ZrO2 particles, as displayed by the new peak at 2θ = 30° and 50°, corresponding to the [1 1 1] and [2 2 0] of zirconia in a tetrahedral amorphous phase (JCPDS card 17-0923) [21,22]. The presence of a dispersed zirconium oxide metastable phase indicates the formation of an aggregation on the support’s surface. This is also related to the involvement of a sol–gel mechanism that occurred through the following general equation:
The rate of a hydrolysis reaction is determined by water content in the dispersion system. Because water is present during the dispersion, hydrolysis may contribute to the possible occurrence of the sol–gel dispersion in ZrO2; the interaction between the Zr4+ cation and water produces a sol–gel in the following equation:
The trend from varying the Zr molar ratio can be expressed as the formation of ZrO2 in heterogeneous form on the pillared montmorillonite surface as function of Zr content; specifically, the higher the Zr loading, the smaller the FWHM of the [1 1 1] reflection obtained, indicating a more crystalline structure. The dispersion also affected the crystallinity of Al-MMT, as indicated by a reduction in the intensity of the [0 0 1] reflection for Al-MMT at an elevated Zr content. The distribution of ZrO2 is related to the interactions between Zr and Al in aluminum-pillared montmorillonite. A previous study described Zr’s exchange into ZSM-5, and the interaction between low ratios of Zr and Al is an exchange interaction and did not affect the crystallinity of the Al-framework [23]. This assumption is also confirmed by the changes in the [0 0 1] reflection intensity, as detected for 40Zr and 60Zr. The presence of the ZrO2 reflection coincides with the decreasing intensity and wider [0 0 1] reflection produced from the interaction between Zr and Al that cannot be accommodated by the exchange process. Zr with higher loadings was then aggregated to form ZrO2 on the surface. The effect of Zr’s loading on Al-MMT also exhibits an adsorption–desorption profile, as shown in Fig. 2.
Fig. 2.

Adsorption–desorption profile of prepared materials.
From the adsorption–desorption pattern, it can be noted that Zr loading reduced the adsorption capacity of Al-MMT. Based on the adsorption data presented in Table 2, the Zr dispersion decreases the specific surface area and pore volume of Al-MMT. The samples, except for 60Zr, have a lower specific surface area relative to Al-MMT due to the ZrO2 blockade against the porous structure of Al-MMT. The formation of ZrO2 aggregates was confirmed by the increasing pore radius with increasing Zr content at a Zr/CEC of more than 40. By enhancing the molar ratio between Zr and CEC to 60, the specific surface area and pore volume will increase. The enhancement of the specific surface area and pore volume most likely arises from the ZrO2 porous formation following the sol–gel hydrolysis of the zirconia precursor. This porous formation was detected easily via a surface morphological analysis conducted on a scanning electron microscope profile of raw montmorillonite, Al-MMT, 10Zr and 60Zr (Fig. 3).
Table 2.
Surface profile data of prepared material compared to raw and aluminum-pillared montmorillonite.
| Sample | Specific surface area (m2/g) | Pore volume (cc/g) | Pore radius (Å) |
|---|---|---|---|
| MMT | 69.87 | 3.33 | 9.76 |
| Al-MMT | 143.92 | 14.75 | 10.09 |
| 10Zr | 126.17 | 12.93 | 14.09 |
| 20Zr | 120.70 | 12.30 | 14.02 |
| 40Zr | 136.96 | 14.00 | 15.28 |
| 60Zr | 144.09 | 14.77 | 16.30 |
Fig. 3.

SEM profile of (a) MMT; (b) Al-MMT; (c) 10Zr; (d) 40Zr; (e) 60Zr.
An important characteristic is the improved catalytic sites that influence the catalysis mechanism. In analogous investigations, theoretically, zirconia contributes to the Lewis acidity enhancement through an external orbital of both metals, while the Brønsted acidity is obtained from protons released during the dehydroxylation during calcination. Increased Lewis acid distribution was also reported for a TiO2 supporting zeolite [25]. This interesting zirconia attachment in the pillared clays requires further investigation to study the physicochemical characteristics and the potential for catalytic applications.
In the phenol hydroxylation reaction, the surface acidity is an important factor that must be provided by the catalyst material. Table 3 lists the changes in the total acidity and acid distribution based on the pyridine-adsorption followed by a FTIR analysis. The FTIR spectra of the materials after pyridine-adsorption are depicted in Fig. 4.
Table 3.
Total acidity and L/B ratio.
| Sample | Total acidity (mmole butylamine/g) | L/B ratio |
|---|---|---|
| Montmorillonite | 0.775 | 0.85 |
| Al-MMT | 1.275 | 1.08 |
| 10Zr | 1.260 | 1.06 |
| 20Zr | 1.350 | 1.12 |
| 40Zr | 1.150 | 1.07 |
| 60Zr | 1.020 | 1.08 |
Fig. 4.

FTIR spectra of Zr/Al-MMTs compared to MMT and Al-MMT.
By comparing the total acidity data presented in Table 3, the increased total surface acidity was attained through the pillarization process, as shown by the higher values for both total acidity and the Lewis to Bronsted ratio of Al2O3-MMT relative to the raw MMT. The presence of aluminum oxide and zirconium oxide inserted into montmorillonite structure contribute to increase surface acidity from outer d-orbital of the metal and therefore the capability of the surface to adsorb basic sites from n-butylamine solution was increased. Comparatively, the change in the total acidity due to ZrO2 dispersion appeared higher than the pillared montmorillonite support at a ratio of Zr/CEC of 10, but the increased ratio does not seem to result in a clear pattern for the total acidity and L/B ratio. These data can be explained by the correlation of the effect of Zr content on the dispersion with the ZrO2 particle formation on the surface, as indicated by the XRD data. The lower total acidity of 10Zr, 40Zr and 60Zr relative to Al-MMT is most likely caused by the presence of aggregated ZrO2 on a surface that might block the porosity of Al-MMT, further decreasing the accessibility and rendering less accessible space for the probe molecules. This result is also verified by the decrease in the specific surface area and pore volume data that was confirmed by an adsorption–desorption profile (Fig. 2 and Table 2).
Comparisons of the Zr/Al-MMT catalytic activity expressed as the kinetics of phenol conversion are presented in Fig. 5. As in similar prior investigations regarding phenol hydroxylation, the reaction involves a free radical mechanism. The interactions between the solid catalysts and H2O2 yields OH• and species via a redox mechanism before the phenol ring is attacked, generating hydroquinone and catechol as the major products [24,25].
Fig. 5.
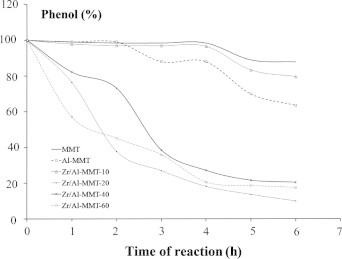
Kinetic curve of phenol hydroxylation at varied catalysts.
From the kinetic simulation of the data and using the parameters of the coefficient of determination (R2), it was concluded that all catalyzed reactions obey a pseudo-first order rate law because of the higher R2 than for any other kinetic order (Table 4).
Table 4.
Kinetic constant of phenol hydroxylation first order reaction rate.
| Catalyst | Parameter of Kinetics Simulation |
||
|---|---|---|---|
| Pseudo-1st Order simulation | R2 of pseudo-2nd Order simulation | R2 of pseudo-3rd Order simulation | |
| MMT | • Coefficient of determination (R2) = 0.8447 | R2 = 0.7099 | R2 = 0.7043 |
| • Initial reaction rate (vo) = 11.18 (% h−1) | |||
| • 1st order constant (k) = 2.149 × 10−2 | |||
| Al-MMT | R2 = 0.9239 | R2 = 0.9104 | R2 = 0.8504 |
| vo = 31.11 (% h−1) | |||
| k = 7.758 × 10−2 | |||
| 10Zr | R2 = 0.8618 | R2 = 0.8569 | R2 = 0.8520 |
| vo = 17.22 (% h−1) | |||
| k = 3.591 × 10−2 | |||
| 20Zr | R2 = 0.9915 | R2 = 0.9757 | R2 = 0.9000 |
| vo = 78.59 (% h−1) | |||
| k = 29.501 × 10−2 | |||
| 40Zr | R2 = 0.9753 | R2 = 0.9732 | R2 = 0.9551 |
| vo = 87.02 (% h−1) | |||
| k = 30.087 × 10−2 | |||
| 60Zr | R2 = 0.9843 | R2 = 0.9696 | R2 = 0.9545 |
| vo = 81.58 (% h−1) | |||
| k = 30.087 × 10−2 | |||
Therefore, Al-MMT has a higher reaction rate than MMT, as indicated by the first order kinetic constant. Except for 10Zr, other samples with varied Zr content have higher reaction rates and kinetic constants relative to Al-MMT. The higher kinetic data are most likely due to Zr acid sites being present, as indicated by the higher L/B ratio. Another trend is that the increased Zr content is not linearly related with the increased reaction rate or kinetic constant. The existence of Zr catalytic sites helps to enhance the interaction between the phenol and catalytic sites, contributing to the increasing kinetics of the reaction mechanism. From the kinetic constant data, the highest activity is achieved by 40Zr. After further comparison with the total acidity and surface area data, the catalytic activity is not linearly related, most likely due to the collaborative role of the physicochemical characteristics of the catalyst material, such as the specific surface area and the presence of Lewis acid sites from Zr dispersion beyond the total acidity parameter. For example, 10Zr has a higher total acidity T but has the lowest rate compared to Al-MMT, with the most probable reason being the lower specific surface area with lower Zr content. The existence of the dispersed ZrO2 most likely cannot affect the interaction of the reactants significantly because the adsorption–desorption mechanism controlled by the surface area is more dominant. In addition, increasing the Zr content enhanced the reaction rate because of the contribution of the ZrO2 particles that act as active sites to generate •OH. The transport of reactants and products in the reaction mechanism is also controlled by the surface sites’ availability. In contrast, further Zr additions reduce the activity, as shown by the lower rate and kinetic constants for 60Zr; even the specific surface area’s values increased. The earlier analysis of the effect of surface acidity on the radical mechanism indicated that the lower total acidity and L/B on the surface might be the main factors affecting the reaction rate. The adsorption–desorption mechanism is an important step in heterogeneous catalysis and is influenced by the intrinsic acidity of the solid catalyst [25–28]. In contrast, 20Zr has a higher total acidity, and L/B has a lower reaction rate. The presence of excess active sites might generate the proper amount of •OH and increase the conversion of phenol; however, at excessively high concentrations, •OH would decompose to form oxygen and not participate in the hydroxylation mechanism, similar to the phenol hydroxylation over Fe/MCM-41 with varied Fe content [25].
Furthermore, the effect of the Zr content on the catalytic activity was studied by evaluating the catalyst’s selectivity. The reaction equation produces three possible compounds. The catalyst’s selectivity may be responsible for the catalyst producing a certain product (Fig. 6). Different Zr contents have varying effects on the product selectivity. Because catechol (CAT) and hydroquinone (HQ) are the first possible products in the mechanism, both compounds are dominant products in all catalyzed reactions, while benzoquinone (BQ) will be produced as further oxidation occurs. In addition, the selectivity for CAT is observed to be higher relative to HQ in all varied catalysts. HQ is more dominant in the product due to the more stable structure compared to CAT. The formation of HQ suggests the presence of surface acidity on the catalysts in that during the catalysis mechanism, the intermediate was form via bonding formation of Lewis acid from either zirconium or oxide sheets of montmorillonite with pi-electron of phenol structure. From the varied Zr content, it is also noted that the 40Zr catalyst provides higher selectivity, producing CAT at longer reaction times (Table 5). The trend for selectivity is similar to the trend for reaction rate, indicating that there was no specified physicochemical characteristic directly related to the reaction mechanism. CAT can be easily produced by all external and internal surface acid sites, while the production of HQ requires a more specific catalyst porosity [23,24].

Fig. 6.

Effect of Zr content at phenol conversion at the phenol to H2O2 mole ratio of 5:1 and 3:1.
Table 5.
Selectivity of reaction products at varied catalyst.
| Catalyst | Time (h) | Selectivity (%) |
||
|---|---|---|---|---|
| HQ | CAT | BQ | ||
| 10Zr | 1 | 12.55 | 87.45 | 0.00 |
| 2 | 11.61 | 88.39 | 0.00 | |
| 3 | 7.47 | 92.53 | 0.00 | |
| 4 | 18.96 | 77.81 | 3.23 | |
| 5 | 20.07 | 79.93 | 0.00 | |
| 6 | 20.63 | 79.37 | 0.00 | |
| 20Zr | 1 | 50.63 | 49.37 | 0.00 |
| 2 | 41.00 | 59.00 | 0.00 | |
| 3 | 38.27 | 60.67 | 1.06 | |
| 4 | 30.79 | 64.59 | 4.62 | |
| 5 | 31.64 | 64.47 | 3.89 | |
| 6 | 29.91 | 66.04 | 4.05 | |
| 40Zr | 1 | 23.77 | 76.23 | 0.00 |
| 2 | 13.20 | 86.80 | 0.00 | |
| 3 | 1737 | 82.63 | 0.00 | |
| 4 | 15.57 | 84.12 | 0.31 | |
| 5 | 15.87 | 83.68 | 0.44 | |
| 6 | 15.97 | 83.82 | 0.21 | |
| 60Zr | 1 | 11.67 | 88.33 | 0.00 |
| 2 | 17.80 | 80.38 | 1.82 | |
| 3 | 15.72 | 82.72 | 1.56 | |
| 4 | 13.66 | 85.20 | 1.13 | |
| 5 | 16.71 | 81.13 | 2.16 | |
| 6 | 16.19 | 82.73 | 1.08 | |
Conclusions
A composite of ZrO2/aluminum-pillared montmorillonite with varied Zr to CEC ratio has been prepared. From varied Zr to CEC ratio, it is found that Zr content affects to the physicochemical characteristics of material as shown by the zirconia crystal formation at higher Zr content, change in specific surface and porosity while total surface acidity and L/B ratio parameter are varied with Zr content. The activity is not linearly correlated with the Zr content but the combination of the presence of ZrO2 in composite, specific surface area and total acidity are responsible factors to the enhanced catalyst activity as acid catalyst during phenol hydroxylation.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The author gratefully acknowledges the use of the facilities and the support of the LIPI Geoteknologi Bandung, Laboratorium Energi Institut Teknologi Sepuluh November Surabaya, Indonesia, and the Chemistry Department of Islamic University of Indonesia.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Varadwaj G.B., Parida K.M. Montmorillonite supported metal nanoparticles: an update on syntheses and applications. RSC Adv. 2013:13583–13593. [Google Scholar]
- 2.Gil A., Gandia L.M., Vicente M.A. Recent advances in the synthesis and catalytic applications of pillared clays. Catal Rev: Sci Eng. 2000;42(1-2) [Google Scholar]
- 3.Mishra T., Mohapatra P., Parida K.M. Synthesis, characterization and catalytic evaluation of iron-manganese mixed oxides pillared clay for VOC decomposition. Appl Catal B: Environ. 2008:279–285. [Google Scholar]
- 4.Das D., Mishra H.K., Parida K.M., Dalai A.K. Preparation, characterisation of Zr, Ti and Zr-Ti mixed oxide pillared montmorillonite and its catalytic activity towards nitration of chlorobenzene. Ind J Chem. 2002;41A:2238–2243. [Google Scholar]
- 5.Mishra T., Parida K. Transition metal pillared clay-5: synthesis, characterisation and catalytic activity of Iron-chromium mixed oxide pillared montmorillonite. Appl Catal A. 1998;174:91–98. [Google Scholar]
- 6.Mishra T., Parida K.M. Transition metal pillared clay: part 4. A compartive study of textural, acidic and catalytic properties of chromia pillared montmorillonite and acid activated montmorillonite. Appl Catal A. 1998;166:123–133. [Google Scholar]
- 7.Singh V., Sapehiyia V., Srivastava V., Kaur S. ZrO2-pillared clay: an efficient catalyst for solventless synthesis of biologically active multifunctional dihydropyrimidinones. Catal Commun. 2006;7(8):571–578. [Google Scholar]
- 8.Ding Z., Kloprogge J.T., Frost R.L., Lu G.Q., Zhu H.Y. Porous clays and pillared clays-based catalysts. part 2 a review of the catalytic and molecular sieve applications. J Porous Mater. 2001;8:273–293. [Google Scholar]
- 9.Kloprogge J.T., Duong D., Frost R.L. A Review of the synthesis and characterization of pillared clays and related porous materials for cracking of vegetable oils to produce biofuels. Environ Geol. 2005;47:967–981. [Google Scholar]
- 10.Pires J., Pinto M.L. Pillared interlayered clays as adsorbents of gases and vapors. In: Gill A., Korili S.A., Trujillano R., Vicente M.A., editors. Pillared clays and related catalysts. Springer; Netherland: 2010. pp. 24–42. [Google Scholar]
- 11.Zuo S., Zhou R., Qi X. Synthesis and characterization of aluminum and Al/REE pillared clays and supported palladium catalysts for benzene oxidation. J Rare Earths. 2011;29(1):52–57. [Google Scholar]
- 12.Carriazo J., Barrault G.E., Tatibouët J.M., Molina R., Moreno Catalytic wet peroxide oxidation of phenol by pillared clays containing Al-Ce-Fe. Water Res. 2005;39(16):3891–3899. doi: 10.1016/j.watres.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Jung H., Paek S., Yoon J., Choy J. Zr K-edge XAS study on ZrO2-pillared aluminosilicate. J Porous Matter. 2007;4:369–377. [Google Scholar]
- 14.Mnasri S., Frini-Srasra N. Influence of aluminium incorporation in the preparation of zirconia-pillared clay and catalytic performance in the acetalization reaction. Clay Miner. 2012;47(4):453–463. [Google Scholar]
- 15.Awate S.V., Waghmode S.B., Agashe M.S. Synthesis, characterization and catalytic evaluation of zirconia-pillared montmorillonite for linear alkylation of benzene. Catal Commun. 2004;5:407–441. [Google Scholar]
- 16.Singh V., Sapeyiyia V., Kad G. Ultrasound and microwave activated preparation of ZrO2-pillared clay composite: catalytic activity for selective, solventless acylation of 1, n-diols. J Mol Catal A. 2004;210(1–2):119–124. [Google Scholar]
- 17.Fatimah I, Narsito K Wijaya. Preparation of zirconium dispersed in aluminium-pillared montmorillonite as catalyst in phenol hydroxylation. In: Kai L, editor. Proceeding of chemistry, biology and environmental engineering (CBEE) 2009, Singapore; IACSIT; 2009. p. 133–7.
- 18.Yurdakoc M., Ackay M., Tonbul A., Yurdakoc K. Acidity of silica-alumina catalysts by amine titration using Hammett indicators and FT-IR study of pyridine adsorption. Turk J Chem. 1999;23:319–327. [Google Scholar]
- 19.Tyagi B., Chudasama C., Jasra R. Characterization of surface acidity of an acid montmorillonite activated with hydrothermal, ultrasonic and microwave techniques. Appl Clay Sci. 2006;31:16–28. [Google Scholar]
- 20.Wang J., Merino J., Aranda P., Galván J., Ruiz-Hitzky E. Reactive nanocomposites based on pillared clays. J Mater Chem. 1998;9:161–168. [Google Scholar]
- 21.Guorong D., Zhang C., Aimei L., Yang X., Lu L., Wang X. Preparation and characterization of mesoporous zirconia made by using a poly (methyl methacrylate) template. Nano Res Lett. 2008;3:118–122. doi: 10.1007/s11671-008-9123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacheen H.S., Iglesia E. Structure of zirconium-exchanged H-ZSM5 prepared by vapor exchange of ZrCl4. Chem Mater. 2007;19:1877–1882. [Google Scholar]
- 23.Reddy E.P., Davydov L., Smirniotis P.G. TiO2-loaded zeolites and MCM-41 in the sonophotocatalytic decomposition of aqueous organic pollutants the role of the support. Appl Catal B: Environ. 2004;42:1–11. [Google Scholar]
- 24.Kulawong S., Prayoonpokarach S., Neramittagapong A., Wittayakun J. Mordenite modification and utilization as supports for iron catalyst in phenol hydroxylation. J Ind Eng Chem. 2011;17:346–351. [Google Scholar]
- 25.Wu C., Kong Y., Gao F., Wu Y., Lu Y., Wang J. Synthesis, characterization and catalytic performance for phenol hydroxylation of Fe-MCM41 with high iron content. Micro Meso Matter. 2008;113:163–170. [Google Scholar]
- 26.Parida K.M., Rath D. Surface characterization and catalytic evaluation of copper-promoted Al-MCM-41 toward hydroxylation of phenol. J Colloid Interface Sci. 2009;340(2):209–217. doi: 10.1016/j.jcis.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Parida K.M., Mallick S. Hydroxylation of phenol over Molybdovanadophosphoric acid modified zirconia. J Mol Catal A: Chem. 2008;279(1):104–111. [Google Scholar]
- 28.Valkaj K.M., Wittine O., Margeta K., Granato T. Phenol oxidation with hydrogen peroxide using Cu/ZSM5 and Cu/Y5 Catalysts. Pol J Chem Technol. 2011;13(3):28–36. [Google Scholar]



