Abstract
The corrosion inhibition of mild steel in 0.5 M H2SO4 solution has been investigated using electrochemical methods, X-ray diffraction (XRD) and scanning electron microscope (SEM). The adsorption and inhibition action of acid corrosion of mild steel using cetyltrimethylammonium bromide (CTABr) and different halides (NaCl, NaBr and NaI) has shown synergetic effect. The results showed that the protection efficiency (P%) has high values at considerable high concentration of CTABr. However, in the presence of the different halides, the P increases dramatically at low concentration of CTABr. Physisorption was proposed from the the values of . The synergism parameter (Sθ) is found to be greater than unity indicating that the enhanced P% caused by the addition of the halides to the CTABr is due to a co-operative adsorption of both species. Corrosion products phases and surface morphology were studied using X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively.
Keywords: Corrosion, Surfactant, Polarization, SEM, XRD
Introduction
Corrosion inhibition of steel is an important issue both from industrial and scientific point of view. For instance in oil production countries worldwide, corrosion protection plays a vital role. Many cases of extensive corrosion have occurred in production tubing, valves, and flow lines from the wellhead to the processing equipment. One of the most important methods to inhibit corrosion of steel is to use adsorption inhibitors for the purpose. This happens in many technological and practical areas such as acid pickling and descaling, petroleum industry storage of chemical in special tanks [1,2]. Among these adsorption inhibitors are inorganic compounds such as chromates and organic compounds bearing sulfur, oxygen or nitrogen heteroatom are widely used as corrosion inhibitors of steel in different media [3–6]. This class of inhibitors exhibits high inhibition efficiency level at considerably low cost, in addition to their availability. Surfactants are one of the important categories which are widely applied as corrosion inhibitors for different metals such as Fe, Al and Cu in different media [7,8].
Surfactants exert their inhibition action through adsorption on the metal surface such that the polar or ionic group (hydrophilic part) attaches to the metal surface while its tail (hydrophobic part) extends to solution. The adsorption of surfactant on metal surface can markedly change the corrosion-resisting property of the metal [9,10]. The strength of adsorption and hence the extent of inhibition is dependent on the nature of the surfactant, nature of the surface and the corrosion medium. Cationic surfactants as a class of the surfactants have been used as corrosion inhibitors for steel both in HCl [4,11] and H2SO4 [12] solutions. The molecular structure of the cationic surfactant affects the mode and extent of adsorption on the metal surface. The ionic head of the cationic surfactant plays a crucial role in the inhibition efficiency. For instance, while cationic surfactant has a pyridinium ring, it has higher inhibition efficiency and tends to chemically adsorbed on the iron surface compared to the tetra methyl. That is to say that the tetra methyl has lower inhibition efficiency than pyridinium. However, in the presence of a halide ion either as a counter ion or in solution, it can help to increase the extent of adsorption due to the well-known synergistic effects [12–14]. This synergism has been reported to be due to the increased surface coverage as a result of ion–pair interactions between an organic cation and the halide anion. Halide ion presents in an inhibiting solution firstly adsorbs on the corroding surface by creating oriented dipoles and thus it facilitates the adsorption of inhibitor cations on the dipoles [15]. In general, cationic surfactants are known for its toxicity and carcinogenicity in addition to their high cost, and hence it is of prime interest to use lower concentration of such inhibitors via using synergism with some ions which are known of its low costs and eco-friendly characteristics such as halides. In the present work, the synergistic inhibition between CTABr and different halide ions in 0.5 M sulfuric acid was investigated by electrochemical methods. The interaction of halide ions with the CTABr molecule and its synergism toward the inhibition of acid corrosion of mild steel is discussed.
Experimental
Mild steel sample used has the following composition (wt.%); 0.07% C, 0.29% Mn, 0.07% Si, 0.012% S, 0.021% P and the remainder iron. Cetyltrimethylammonium bromide (CTABr) and sodium halides were obtained from Aldrich and used as received. The solution of 0.5 M H2SO4 was prepared by dilution of AR grade 96% H2SO4. Stock solutions of surfactant and halides were prepared in 0.5 M H2SO4 and the desired concentrations were obtained by appropriate dilution.
Electrochemical measurements were carried out in a conventional three-electrode cell with a platinum counter electrode (CE) and a Hg/Hg2SO4/(1.0 M) [E = 0.674 V (NHE)] coupled to a fine Luggin capillary as the reference electrode (RE). In order to minimize the ohmic contribution, the Luggin capillary was kept close enough to the working electrode (WE). The latter was fitted into a glass tube of proper internal diameter by using epoxy resins. The WE surface area of 0.5 cm2 was abraded with emery paper (grade 320–500–800–1000–1200) on test face, rinsed with distilled water, degreased with acetone, and dried with a cold air stream. Before measurements the electrode was immersed in the test solution at open circuit potential (OCP) for 15 min at 25 °C or until the steady state is obtained. All electrochemical measurements were carried out using an EG&G Princeton Applied Research (model 273A) potentiostat/galvanostat controlled by m352 electrochemical analysis software. The potential of potentiodynamic polarization curves was done from a potential of −250 mV vs. OCP, to 250 mV vs. OCP at a scan rate of 2 mV/s. Current densities were calculated on the basis of the apparent geometrical surface area of the electrode.
A computer controlled X-ray diffraction technique, PANalytical model X’pert pro powder was used to scan the corrosion products between 0° and 80° 2θ with copper Kα radiation (Ni filter) at a rating of 45 kV and 40 mA. The dried corrosion products were collected, crushed into a fine powder and used for XRD analysis for determining the nature of film present on the metal surface. Surface morphology of mild steel samples and protective films were examined by scanning electron microscopy (FEI Quanta 3D 200i). The mild steel samples with surface area of 0.5 cm2 was abraded with emery paper (grade 320–500–800–1000–1200) on both faces, rinsed with distilled water. Before examination the sample was immersed in the test solution for 30 min at 25 °C.
Results and discussion
Open circuit potential
The variation of the open circuit potential (OCP) of mild steel was followed as a function of time as shown in Fig. 1 in different combinations of CTABr and halide ions in 0.5 M H2SO4 solutions. Curve 1 is for the blank (0.5 M H2SO4) and curve 2 is for 7 × 10−6 M CTABr (in 0.5 M H2SO4). Curves 3–5 are for 7 × 10−6 M CTABr (in 0.5 M H2SO4) + 0.1 M halide ions (NaCl (curve 3), NaBr (curve 4) and NaI (curve 5)). While in the presence of 7 × 10−6 M CTABr (curve 2) or in the presence of both the CTABr and 0.1 M Cl− (curve 3) drift the steady state Ecor to positive values (with respect to blank (0.5 M H2SO4)), the presence both CTABr and 0.1 M Br− (curve 4) or I− (curve 5) drift Ecor to more positive values with respect to the blank (0.5 M H2SO4). However, the general shape of the OCP-time curves does not change. According to some literature reports [16], the classification of an inhibitor as an anodic or cathodic type inhibitor is feasible when the shift in the corrosion potential Ecor is at least ±85 mV as compared to the blank solution. We can note that the shift in Ecor on adding the CTABr is less than 40 mV revealing that the CTABr and CTABr/halide systems affects both the anodic dissolution of iron and the hydrogen evolution reaction and acts as mixed type inhibitor systems. Also, in Fig. 1 by adding NaCl to the CTABr (curve 3) the same trend as that in the presence of the CTABr only is obtained. When NaBr (curve 4) and NaI (curve 5) are added, the steady state is obtained quickly and the shift in Ecor is less than in absence of NaCl indicating that CTABr/NaBr and CTABr/NaI systems are more inhibiting systems than CTABr/NaCl system. The results clearly indicate that the shifting to noble direction of potential is in the order I− > Br− > Cl−.
Fig. 1.

Open circuit potential, EOCP – time relations for mild steel immersed in (1) 0.5 M H2SO4, (2) 7 × 10−6 M CTABr, (3–5) 7 × 10−6 M CTABr + (0.1 M of (3) NaCl, (4) NaBr and (5) NaI, respectively).
Polarization studies
Fig. 2 shows the polarization curves for mild steel in 0.5 M H2SO4 (curve 1), containing 7 × 10−6 M CTABr (curve 2), 0.10 M halide (curve 3) and both (0.1 M halide + 10−5 M CTABr) (curve 4). Panels A–C show the case of Cl−, Br− and I−, respectively. It is clear that the mild steel corrosion is slightly inhibited in the presence of either a small concentration of CTABr (7 × 10−6 M curve 2) or halides (0.10 M curve 3). In the presence of both species (curve 4), however, both anodic and cathodic branches are dramatically shifted to lower currents. The presence of halide ions only (curve 2) causes slight shifts in both anodic and cathodic currents i.e., a slight decrease in the corrosion rate. This could be ascribed to adsorption of halide over the corroded surface [17]. In other words, both cathodic and anodic reactions of mild steel are slowly retarded by halides. In the presences of iodide only (panel C, curve 3) the decrease in both anodic and cathodic currents is significant. Generally, the adsorbability of anions is related to the degree of hydration. The less hydrated ion is preferentially adsorbed on the metal surface. The ease of adsorption (greater protection efficiency) shown in the case of the iodide ions may be due to its less degree of hydration. The protective effect of halide ions is found to be in the same order as that of adsorption ability.
Fig. 2.

Polarization curves for mild steel in (1) 0.5 M H2SO4 (blank), (2) 7 × 10−6 M CTABr, (3) 0.1 M halide and (4) 7 × 10−6 M CTABr + 0.1 M of halide; panel (A) for NaCl, panel (B) for NaBr and panel (C) for NaI.
Table 1 lists important corrosion parameters such as the free corrosion potential (Ecor), corrosion current density (icor), slope of the cathodic branch (βc) and slope of the anodic branch (βa). The cathodic Tafel slope (βc) for mild steel in the absence and presence of different systems does not change significantly indicating that all systems does not change the mechanism of the HER, and the corrosion is rather inhibited by blocking of the iron surface by simple adsorption process. We can also note that the corrosion current decreases significantly in the presence of CTABr and the halide ions specially the I− ion.
Table 1.
Electrochemical parameters for the corrosion of mild steel in 0.5 M H2SO4 in the absence and presence of 7 × 10−6 M and/or 0.10 M halide.
| System | Ecor/V (Hg/Hg2SO4) | icor/mA cm−2 | βa/V (decade)−1 | βc/V (decade)−1 | Picor% |
|---|---|---|---|---|---|
| Blank | −0.923 | 0.969 | 0.219 | −0.183 | |
| CTABr | −0.926 | 0.870 | 0.207 | −0.222 | 10.2 |
| NaCl | −0.928 | 0.660 | 0.247 | −0.221 | 31.9 |
| NaBr | −0.926 | 0.583 | 0.239 | −0.216 | 39.8 |
| NaI | −0.916 | 0.313 | 0.088 | −0.144 | 67.7 |
| CTABr/NaCl | −0.928 | 0.337 | 0.185 | −0.190 | 65.3 |
| CTABr/NaBr | −0.921 | 0.265 | 0.146 | −0.171 | 72.6 |
| CTABr/NaI | −0.880 | 0.049 | 0.075 | −0.172 | 94.8 |
In order to demonstrate the different behavior of the halides we may use one concentration of CTABr and the same halide concentration in one figure. Fig. 3 shows the polarization curves for mild steel in 0.5 M H2SO4 (curve 1), containing 7 × 10−6 M CTABr (curve 2), 7 × 10−6 M CTABr + 0.1 M halide ions (NaCl (curve3), NaBr (curve 4) and NaI (curve 5)). Where both anodic and cathodic branches are dramatically shifted to lower currents which indicate there is a synergism between cationic surfactant and anionic halides in order of I− ≫ Br− > Cl−.
Fig. 3.

Polarization curves for mild steel in (1) 0.5 M H2SO4 (blank), (2) 7 × 10−6 M CTABr, (3–5) 7 × 10−6 M CTABr + (0.1 M of (3) NaCl, (4) NaBr and (5) NaI, respectively).
Protection efficiency
The protection efficiency, P% is given by;
| (1) |
where icor1 and icor2 are corrosion current densities in the absence and presence of the inhibitor, respectively. Fig. 4 shows the effects of the CTABr concentration on the protection efficiency (P%). The dependence of P% on CTABr concentration is of a sigmoid shape. P% increases with the CTABr concentration until it reaches a certain concentration which corresponds to the critical micelles concentration (CMC) (CMC of CTABr = 1 × 10−4 M at 30 °C) [18]. The inhibition is attributed to the adsorption of the surfactant on the iron surface. At [CTABr] about 10−5 M, the monomers of CTABr adsorb at the surface individually with a low percent coverage. As the concentration increases (in the range between 1 × 10−4 M and up to the start of the plateau) the amount adsorbed increases leading to a higher degree of coverage and consequently higher corrosion inhibition. Adsorption is enhanced due to the inter-hydrophobic chain interaction. This may be attributed to the formation of a bimolecular layer of surfactant through the interaction of the hydrocarbon chains by tail–tail orientation at the electrode–electrolyte interface [19]. Such interaction assists the formation of a thin film of surfactant molecules at the iron surface. This film is of hydrophobic nature due to anchoring of the hydrophobic chains into the solution. At higher inhibitor concentration near the CMC, a plateau is obtained. At this high concentration the surfactant molecules tend to aggregate and form micelles rather than adsorption on the iron surface [20].
Fig. 4.

Effects of CTABr concentration on P% for mild steel in 0.5 M H2SO4.
Polarization curves of mild steel were collected (data are not shown here) in 0.5 M H2SO4 at different concentrations of the halide ions in the presence of 7 × 10−6 M CTABr. Those polarization curves were used to determine the protection efficiency as shown in Fig. 5. The figure demonstrates the dependence of the protection efficiency, P% on the concentration of the halide. Using data in Table 1 of icor, the values of P% in the presence of CTABr only (halide-free solution) is 10.2% at of 7 × 10−6 M CTABr.
Fig. 5.

Effects of halide ion concentration on P% for mild steel at 7 × 10−6 M CTABr in 0.5 M H2SO4.
Inspection of Fig. 5 reveals that the inhibition efficiency increases with the increase in the halide concentration reaching a constant value depending on the halide ions. It is 75%, 95%, and 95% for Cl−, Br− and I−, respectively. In the latter ions (Br− and I−) almost complete suppression of corrosion is obtained. In the presence of a low concentration of the CTABr (7 × 10−6 M) a significant increase in the protection efficiency was obtained upon addition of the different halides. Briefly, P% increases from 10.2% in the presence of only CTABr to 76.1% in the presence of (7 × 10−6 M CTABr + 0.50 M Cl−). Generally, the P% in the presence of similar concentration of the halide decreases in the order; I− > Br− > Cl−.
Note that the P% in the presence of CTABr alone (i.e., halide-free solution) is 10.2% at 7 × 10−6 M CTABr (see Table 1). Note that the [Br−] produced from the dissociation of the [CTABr] (assuming 100% dissociation) is the same as that of CTABr. Accordingly, this Br− ion coming from the CTABr molecules will be added to the intentionally added halide concentrations. However, inspection of the values of P% in Fig. 5, in case of Cl− and I− (added to CTABr), we can see that the P% values are much higher than in the case of using CTABr alone. In case of the Br− the start of the increase in P% is when the added [Br−] is higher than that coming from the dissociation of the CTABr itself. Khamis [21] have reported that the addition of halide salt to sulfuric acid solution containing cetylpyridinium chloride causes a synergistic or cooperative effect which inhibits iron corrosion. Halide ions have been shown to inhibit the corrosion of some metals in strong acids and this effect depends on the ionic size and charge, the electrostatic field set up by the negative charge of the anion on the adsorption site and the nature and concentration of the halide ion. From the observed trend of increase in the protective action in the order I− > Br− > Cl−, it is likely that the radii and the electronegativity of the halide ions has a profound influence on the adsorption process. Electronegativity increases from I− to Cl− (I− = 2.5, Br− = 2.8, Cl− = 3.0) while atomic radius decreases from I− to Cl− (I− = 135 pm, Br− = 114 pm, Cl− = 90 pm) [17]. The iodide ion is more predisposed to adsorption than the bromide and chloride ions. Stabilization of the adsorbed halide ions by means of interaction with the CTABr leads to greater surface coverage θ and thereby greater protection efficiency.
Synergism
It is likely that the adsorption of a cationic surfactant is enhanced by increasing the negative charge density on the metal surface. Thus the pre-adsorption of halide ions could enhance the adsorption of the cationic surfactant due to ion–pair interactions between the CTABr molecules and the halide ions, resulting in what is the so-called inhibition synergism. The discussion of the synergism factor here may help to distinguish the difference in performance of the combination of the CTABr and different halides. In this part, the effect of halide concentration on the inhibition efficiency of CTABr is studied. This interaction can be quantized by a parameter called synergism parameter (Sθ) [22,23] which is defined as,
| (2) |
where θ1+2 = (θ1 + θ2) − (θ1 θ2), θ1 and θ2 are the degrees of surface coverage in presence of the halide ion and the CTABr, respectively, and is the degree of surface coverage in the presence of both species. Note that the degree of surface coverage, Sθ was determined from the polarization data (θ = P%/100). Sθ approaches unity when no interaction takes place between the inhibitor molecules and the halide ion. At Sθ > 1, a synergistic effect is obtained as a result of a co-operative adsorption. In case of Sθ < 1, antagonistic behavior prevails due to a competitive adsorption [24]. Fig. 6 shows the effects of the halides ion concentration on Sθ estimated in the presence of 0.5 M H2SO4 solution containing 7 × 10−6 M CTABr. The Sθ values are found to be higher than unity, suggesting the synergistic action of halide ion with the CTABr. The above results reveal that CTABr can act as an effective inhibitor in 0.5 M H2SO4 solution even at low concentration in the presence of halide ions. The synergism parameter, Sθ increases with the halide concentrations until it reaches a maximum value after which it decreases or remains constant. This may be due to the adsorption of the CTABr molecules on the metal surface via the nitrogen atom which might compete with the halide ion leading to lesser synergistic effect at higher concentrations of the halide ion [15,21]. Also, the obtained Sθ is in the order Cl− < I− < Br−. Although the highest P% obtained in the case of iodide with CTABr, the highest Sθ is obtained in the case of bromide. This is due to the fact that the inhibition efficiency in the presence of iodide alone Fig. 2 (panel C) is relatively high, compared with the bromide and chloride (panels B and A, respectively). While some literatures [25,26] showed order of Sθ as Cl− < Br− < I−, others [27] reported different orders that depends on the nature of the metal surface and solution composition.
Fig. 6.

Effect of halide ions concentration on the synergism parameter Sθ obtained in the presence of 7 × 10−6 M CTABr.
Adsorption isotherms
Adsorption isotherms can provide the basic information on the interaction between the inhibitor and the mild steel surface. Attempts were made to fit the experimental data to various isotherms including Frumkin, Langmuir, Temkin, Freundlich, Bockris–Swinkels, and Flory–Huggins isotherms. It has been found that the experimental results in this study for both CTABr and CTABr/halide ions systems fit with Temkin isotherm and the plots are presented in Fig. 7 for the surfactant alone (inset) and the CTABr/halides systems. Temkin isotherm is given by Eq. (3); [28–31]:
| (3) |
where θ is the degree of surface coverage, C is the inhibitor concentration, a is the molecular interaction parameter and K is the equilibrium constant of the adsorption process. Plots of θ against log [halide] are depicted in Fig. 7 at 7 × 10−6 M CTABr. The inset shows the plot for CTABr only. That is to say the unshared electron pairs in nitrogen atom could interact with the d-orbitals of the iron to form a protective adsorbed film. The adsorption and thermodynamic parameters deduced from the above plots are listed in Table 2. It can be deduced that there is a repulsion force in the compact adsorption layer since a < 0. The values of a in all CTABr/halide systems are negative indicating that repulsion exists in the compact adsorption layer. It is generally known that K denotes the strength between the adsorbate and adsorbent. Large values of K imply more efficient adsorption and hence better protection efficiency. The standard adsorption free energy was estimated using the following equation [32,33]:
| (4) |
Fig. 7.

Temkin adsorption isotherm for CTABr (Inset) and CTABr/halide ions systems.
Table 2.
Calculated values obtained from Temkin isotherm for CTABr and CTABr/halide ion systems in 0.5 M H2SO4.
| System | a | K/L mol−1 | R2 | kJ/mol |
|---|---|---|---|---|
| CTABr | −1.30 | 1.60 × 104 | 0.9922 | −33.9 |
| 7 × 10−6 M CTABr + NaCl | −14.75 | 2.42 × 107 | 0.9918 | −52.1 |
| 7 × 10−6 M CTABr + NaBr | −10.36 | 2.61 × 107 | 0.9907 | −52.2 |
| 7 × 10−6 M CTABr + NaI | −8.12 | 3.50 × 107 | 0.9909 | −53.0 |
Inspection of Table 2 reveals that K and values are in the order;
The values of in Table 2, indicate mixed mode of adsorption, while CTABr may adsorb in a physisorption mode and the specific adsorption of halide ions is of chemisorptions mode. Generally, values of up to −20 kJ/mol are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption) in case of CTABr alone, while those more negative than 40 kJ/mol involve sharing or transfer of electrons from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (chemisorption) in case of CTABr/halide systems [34,35]. The above values of are consistent with the results of the protection efficiency.
Analysis of X-ray diffraction patterns
The X-ray diffraction patterns of the film formed on surface of the mild steel specimens immersed in various test solutions are given in Fig. 8. (a–c for blank, 1 × 10−3 M CTABr and 7 × 10−6 M + 0.10 M NaCl, respectively.)
Fig. 8.

XRD pattern obtained on the surface film formed on mild steel at the end of 10 days in different environment. Curves: (a) blank, (b) 1 × 10−3 M CTABr, (c) 7 × 10−6 M CTABr + 0.10 M NaCl.
Fig. 8a shows the XRD pattern for the material in blank solution, and it displays the presence of different species of 91% rozenite (FeSO4·4H2O) and 9% melanterite (FeSO4·7H2O) phases, respectively (JCPDS No. 35-1360 and JCPDS No. 25-614) that are commonly formed in sulfuric acid medium as corrosion product [36]. Fig. 8b and c display the spectrum for the rust material studied after the immersion in 1 × 10−3 M CTABr and 7 × 10−6 M CTABr + 0.10 M NaCl, respectively. It is observed that the peaks due to iron sulfates (rozenite and melanterite) showed less intensity than that corresponding to the material in blank (Fig. 8a). Apparently, the sulfated anion is tied to the ionic liquid via the cathodic ion, and in this way iron dissolution of steel is mitigated and sulfate desorption is hindered [36].
SEM examination
SEM images are shown in Fig. 9 for mild steel taken after 30 min immersion in (a) 0.5 M H2SO4, (b) 7 × 10−6, (c) 2 × 10−4 and (d) 1 × 10−3 M CTABr. Close examinations of SEM image taken in the absence of the inhibitor reveals that the specimen surface was strongly damaged with deep cavities (Fig. 9a). In the presence of different concentration of CTABr, the steel specimen has a better morphology with increase in the CTABr concentration (Fig. 9b and c) and the best smooth surface is obtained at higher concentration of the CTABr (Fig. 9d). Fig. 10 shows the SEM images for mild steel after 30 min immersion in (a) 0.5 M H2SO4, (b) 7 × 10−6 M CTABr, (c) 0.10 M NaCl and (d) 7 × 10−6 M CTABr + 0.10 M NaCl. In the presence of combined CTABr + NaCl (Fig. 10d), the steel specimen has a better morphology and smooth surface compared with that of the surface immersed in either CTABr or NaCl solutions (Fig. 10b and c, respectively) with few small notches. This indicates that the combined use of CTABr and NaCl hinders the dissolution of iron and thereby reduces the rate of corrosion, and it reveals good protection against corrosion. The less damage of mild steel surface when dipped in 0.5 M H2SO4 containing either high concentration of the CTABr (Fig. 9d) or CTABr and NaCl (Fig. 10d) might be due to the specific adsorption of Cl− ions on the mild steel which facilitates the adsorption of CTABr molecules.
Fig. 9.

SEM images of mild steel after 30 min immersion in (a) 0.5 M H2SO4, (b) 7 × 10−6, (c) 2 × 10−4 and (d) 1 × 10−3 M CTABr.
Fig. 10.
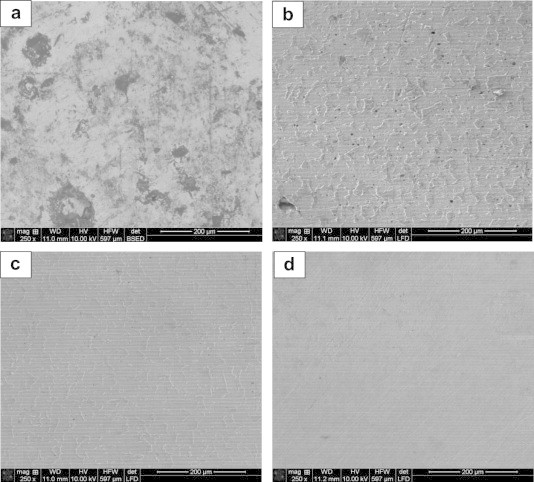
SEM images of mild steel after 30 min immersion in (a) 0.5 M H2SO4, (b) 7 × 10−6 M CTABr, (c) 0.10 M NaCl and (d) 7 × 10−6 M CTABr + 0.10 M NaCl.
Explanation of inhibition
The adsorption of inhibitor on iron/solution interface is affected by the chemical structures of the inhibitors, the nature of the charged metal surface and the distribution of charge over the whole inhibitor molecules. The CTABr molecule can be presented as a cationic surfactant; the organic part (3-methyl ammonium group) CTA+ is the cation and the inorganic part Br− is the anion. Accordingly, the CTABr inhibitor exerts its inhibition action by adsorption of the organic part on the iron surface via the 3-methyl ammonium group on the metal surface while the hydrophobic part C16H33– extends to the solution forming a hydrophobic barrier that protects metal surface from aqueous attack. The mode of adsorption of CTABr and its synergism with halides may be discussed in light of potential of zero charge. In the present case Ecor of the iron, under the present conditions, equals −512 mV (SCE) and in view of the fact that the potential of zero charge for iron in H2SO4 is −550 mV (SCE) [37], it is expected that the iron surface is positively charged. The specific adsorption of the Br− ion and to lower extent of adsorption of ion creates an excess negative charge on the metal surface and favors more adsorption of the cations [23]. In other words, there may be a synergism between anions (Br− and ) and the cationic inhibitor. It is generally accepted that the Br– ions have stronger tendency to adsorb more than the ions [23], and hence the electrostatic influence may be the reason for the increased protective effect in the halide-containing solution [38]. Thus a co-operative adsorption exists between halide ions and CTABr dominates over competitive adsorption. According to co-operative mechanism, halide ions are initially adsorbed on anode of metal surface and then CTABr cations are adsorbed on the layer of halide ions by coulombic attraction forming ion pairs on mild steel surface [28].
Conclusions
Protection efficiency obtained in the presence of CTABr with the coexistence of halides increases in the order: Cl− > Br− > I−, which seems to indicate that the radii and the electronegativity of the halide ions play a significant role in the adsorption process. The values of Sθ (synergistic parameter) are greater than unity showing the corrosion inhibition brought about by CTABr in combination with the halides is synergistic in nature and co-operative adsorption between halides and CTABr prevails over competitive adsorption. CTABr adsorption is physisorption in nature as revealed from values, while the adsorption of CTABr/halide ions systems is chemisorption in nature. for CTABr/halide ions systems is larger than CTABr alone showing that a CTABr/halide ions system is strongly adsorbed than CTABr alone. XRD shows the absence of corrosion product in both cases of high CTABr concentration or low CTABr concentration with halide ion. SEM shows that the best morphology and smooth surface is obtained in both cases of high CTABr concentration or low CTABr concentration with halide ion.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Rosenfeld I.L. Springer-Verlag; London: 2004. Corrosion inhibitors. p. 326–8. [Google Scholar]
- 2.Mansfeld F. Marcel Dekker; New York: 1978. Corrosion inhibitors. p. 742–4. [Google Scholar]
- 3.Emregu’I K.C., Akay A.A., Atakol O. The corrosion inhibition of steel with Schiff base compounds in 2 M HCl. Mat Chem Phys. 2005;93:325–329. [Google Scholar]
- 4.Qiu L.G., Xie A.J., Shen Y.H. The adsorption and corrosion inhibition of some cationic gemini surfactants on carbon steel surface in hydrochloric acid. Corros Sci. 2005;47:273–278. [Google Scholar]
- 5.Noor E.A. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros Sci. 2005;47:33–55. [Google Scholar]
- 6.Atia A.A., Saleh M.M. Inhibition of acid corrosion of steel using cetylpyridinium chloride. J Appl Electrochem. 2003;33:171–177. [Google Scholar]
- 7.Lake D.L. Approching environmental acceptability in cooling water corrosion inhibition. Corros. Prevent. Control. 1988:113–114. [Google Scholar]
- 8.Sastri V.S. John Wiley and Sons; NY: 1998. Corrosion inhibitors; principles and applications. p. 198–202. [Google Scholar]
- 9.Free M.L. Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acidic medium. Corros Sci. 2002;44:2865–2870. [Google Scholar]
- 10.Li X., Deng S., Fu H. Adsorption and inhibitive action of hexadecylpyridinium bromide on steel in phosphoric acid produced by dihydrate wet method process. J Appl Electrochem. 2011;41:507–517. [Google Scholar]
- 11.Zucchi F., Trabanelli G., Brunoro G. The influence of the chromium content on the inhibitive efficiency of some organic compounds. Corros Sci. 1992;33:1135–1139. [Google Scholar]
- 12.Schweinsberg D.P., Ashworth V. The inhibition of the corrosion of pure iron in 0.5 M sulphuric acid by n-alkyl quaternary ammonium iodides. Corros Sci. 1988;28:539–545. [Google Scholar]
- 13.Popova A., Sokolova E., Raicheva S., Christov M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci. 2003;45:33–58. [Google Scholar]
- 14.Larabi L., Harek Y., Traisnel M., Mansri Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1 M HCl. J Appl Electrochem. 2004;34:833–839. [Google Scholar]
- 15.Oguzie E.E., Li Y., Wang F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J Colloid Interface Sci. 2007;310:90–98. doi: 10.1016/j.jcis.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Riggs OL Jr. Corrosion inhibitors, 2nd ed. Nathan Houston TX; 1973. p. 274–6.
- 17.Tennet R.M., editor. vol. 56. Oliver and Boyd; Edinburgh: 1978. pp. 478–479. (Science data book). [Google Scholar]
- 18.Myer D. VCH; New York: 1988. Surfactants science and technology. [Google Scholar]
- 19.Elachouri M., Hajji M.S., Kertit S., Essassi E.M., Salem M., Coudert R. Some surfactants in the series of 2-(alkyldimethylammonio) alkanol bromides as inhibitors of the corrosion of iron in acid chloride solution. Corros Sci. 1995;37:381–389. [Google Scholar]
- 20.Murakawa T., Kato T., Nagaura S., Hackerman N. A contribution to the understanding of the synergistic effect of anions for the corrosion inhibition of Fe by amines. Corros Sci. 1968;8:483–489. [Google Scholar]
- 21.Khamis A., Saleh M.M., Awad M.I. Synergistic inhibitor effect of cetylpyridinium chloride and other halides on the corrosion of mild steel in 0.5 M H2SO4. Corros Sci. 2013;66:343–349. [Google Scholar]
- 22.Bockris J.O.M., Yang B. The mechanism of corrosion inhibition of iron in acid solution by acetylenic alcohols. J Elecrochem Soc. 1991;138:2237–2252. [Google Scholar]
- 23.Deng S., Li X., Fu H. Alizarin violet 3B as a novel corrosion inhibitor for steel in HCl, H2SO4 solutions. Corros Sci. 2011;53:3596–3602. [Google Scholar]
- 24.Umoren S.A., Ogbobe O., Igwe I.O., Ebenso E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci. 2008;50:1998–2006. [Google Scholar]
- 25.Asefi D., Arami M., Mahmoodi N.M. Electrochemical effect of cationic gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid medium. Corros Sci. 2010;52:794–800. [Google Scholar]
- 26.Jeyaprabha C., Sathiyanarayanan S., Muralidharan S., Venkatachari G. Corrosion inhibition of iron in 0. 5 mol L−1 H2SO4 by halide ions. J Braz Chem Soc. 2006;17:61–67. [Google Scholar]
- 27.Jeyaprabha C., Sathiyanarayanan S., Venkatachari G. Influence of halide ions on the adsorption of diphenylamine on iron in 0.5 M H2SO4 solutions. Electrochim Acta. 2006;51:4080–4088. [Google Scholar]
- 28.Sahin M., Bilgic S., Yılmaz H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl Surf Sci. 2002;195:1–7. [Google Scholar]
- 29.Bilgic S., Calıskan N. The effect of N-(1-toluidine) salicylaldimine on the corrosion of austenitic chromium–nickel steel. Appl Surf Sci. 1999;152:107–114. [Google Scholar]
- 30.Priya A.R.S., Muralidharam V.S., Subramania A. Development of novel acidizing inhibitors for carbon steel corrosion in 15% boiling hydrochloric acid. Corrosion. 2008;64:541–552. [Google Scholar]
- 31.Mu G.N., Li X.M., Liu G.H. Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0.5 M sulfuric acid. Corros Sci. 2005;47:1932–1952. [Google Scholar]
- 32.Li W.H., He Q., Zhang S.T., Pei C.L., Hou B.R. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem. 2008;38:289–295. [Google Scholar]
- 33.Cano E., Polo J.L., Iglesia A.L., Bastidas J.M. A study on the adsorption of benzotriazole on copper in hydrochloric acid using the inflection point of the isotherm. Adsorption. 2004;10:219–225. [Google Scholar]
- 34.Bensajjay E., Alehyen S., Achouri M., Kertit S. Corrosion inhibition of steel by 1-phenyl 5-mercapto 1,2,3,4-tetrazole in acidic environments (0.5 M H2SO4 and 1/3 M H3PO4) Anti-Corros Meth Mater. 2003;50:402–409. [Google Scholar]
- 35.Bentiss F., Lebrini M., Lagrenee M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci. 2005;47(12):2915–2931. [Google Scholar]
- 36.Likhanova N.V., Dominguez-Aguilar M.A., Olivares-Xometl O., Nava-Entzana N., Arce E., Dorantes H. The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros Sci. 2010;52(6):2088–2097. [Google Scholar]
- 37.Roy S.C., Roy S.K., Sircar S.C. Critique of inhibitor evaluation by polarisation measurements. Brit Corros J. 1988;32:102–104. [Google Scholar]
- 38.Lebrini M., Lagrenee M., Traisnel M., Gengembre L., Vezin H., Bentiss F. Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: electrochemical, X-ray photoelectron spectroscopy and theoretical studies. Appl Surf Sci. 2007;253(23):9267–9276. [Google Scholar]



