Table 3.
Model parameters for hepatitis C virus pharmacokinetic–pharmacodynamic model
| Parameter | Value |
|---|---|
| p (fixed)* | 100 |
| d (day−1) (fixed)* | 0.001 |
| e (ml day−1) (fixed) * | 1E-07 |
| s (ml−1 day−1) (fixed)* | 20 000 |
| βka (day−1) | 0.80 |
| βke (day−1) | 0.15 |
| βVd (ml) | 100 000 |
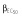 (μg ml−1) (μg ml−1) |
0.00012 |
| βn | 2 |
| βδ (day−1) | 0.20 |
| βc (day−1) | 7 |
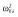 |
0.25 |
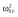 |
0.25 |
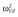 |
0.25 |
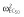 |
0.25 |
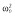 |
0.25 |
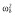 |
0.25 |
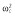 |
0.25 |
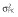 |
0.04 |
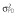 |
0.04 |
Abbreviations are as follows: c, rate constant of elimination of viral particles; EC50, drug concentration in the blood at which the drug is 50% effective; ka, rate constant of absorption; ke, rate constant of elimination; n, Hill coefficient; Vd, volume of distribution; β, fixed effects; δ, rate constant of elimination of infected cells; 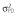 , residual variance for the pharmacodynamic response;
, residual variance for the pharmacodynamic response; 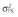 , residual variance for the pharmacokinetic response; ω2, interindividual variance.
, residual variance for the pharmacokinetic response; ω2, interindividual variance.
Parameters are defined in Examples section.
